Extracellular calcium controls background current and neuronal excitability via an UNC79-UNC80-NALCN cation channel complex
- PMID: 21040849
- PMCID: PMC2987630
- DOI: 10.1016/j.neuron.2010.09.014
Extracellular calcium controls background current and neuronal excitability via an UNC79-UNC80-NALCN cation channel complex
Abstract
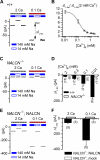
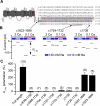
In contrast to its extensively studied intracellular roles, the molecular mechanisms by which extracellular Ca(2+) regulates the basal excitability of neurons are unclear. One mechanism is believed to be through Ca(2+)'s interaction with the negative charges on the cell membrane (the charge screening effect). Here we show that, in cultured hippocampal neurons, lowering [Ca(2+)](e) activates a NALCN channel-dependent Na(+)-leak current (I(L-Na)). The coupling between [Ca(2+)](e) and NALCN requires a Ca(2+)-sensing G protein-coupled receptor, an activation of G-proteins, an UNC80 protein that bridges NALCN to a large novel protein UNC79 in the same complex, and the last amino acid of NALCN's intracellular tail. In neurons from nalcn and unc79 knockout mice, I(L-Na) is insensitive to changes in [Ca(2+)](e), and reducing [Ca(2+)](e) fails to elicit the excitatory effects seen in the wild-type. Therefore, extracellular Ca(2+) influences neuronal excitability through the UNC79-UNC80-NALCN complex in a G protein-dependent fashion.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Sodium leak channels in neuronal excitability and rhythmic behaviors.Neuron. 2011 Dec 22;72(6):899-911. doi: 10.1016/j.neuron.2011.12.007. Neuron. 2011. PMID: 22196327 Free PMC article. Review.
-
Structure and mechanism of NALCN-FAM155A-UNC79-UNC80 channel complex.Nat Commun. 2022 May 12;13(1):2639. doi: 10.1038/s41467-022-30403-7. Nat Commun. 2022. PMID: 35550517 Free PMC article.
-
The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm.Cell. 2007 Apr 20;129(2):371-83. doi: 10.1016/j.cell.2007.02.041. Cell. 2007. PMID: 17448995
-
A uniquely adaptable pore is consistent with NALCN being an ion sensor.Channels (Austin). 2013 Mar-Apr;7(2):60-8. doi: 10.4161/chan.23981. Epub 2013 Feb 26. Channels (Austin). 2013. PMID: 23442378 Free PMC article.
-
The NALCN ion channel is a new actor in pancreatic β-cell physiology.Islets. 2010 Jan-Feb;2(1):54-6. doi: 10.4161/isl.2.1.10522. Islets. 2010. PMID: 21099296 Review.
Cited by
-
Thallium's Threat to Aquatic Life: Stage-Specific Toxicity in Zebrafish Embryos and Larvae.Environ Health (Wash). 2024 Feb 7;2(3):114-125. doi: 10.1021/envhealth.3c00196. eCollection 2024 Mar 15. Environ Health (Wash). 2024. PMID: 39473815 Free PMC article.
-
Cadherin 16 promotes sensory gating via the endocrine corpuscles of Stannius.bioRxiv [Preprint]. 2024 Sep 25:2024.09.23.614609. doi: 10.1101/2024.09.23.614609. bioRxiv. 2024. PMID: 39386705 Free PMC article. Preprint.
-
Astrocyte Ca2+ in the dorsal striatum suppresses neuronal activity to oppose cue-induced reinstatement of cocaine seeking.Front Cell Neurosci. 2024 Aug 29;18:1347491. doi: 10.3389/fncel.2024.1347491. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39280793 Free PMC article.
-
Systematized Serendipity: Fishing Expeditions for Anesthetic Drugs and Targets.Anesthesiology. 2024 Nov 1;141(5):997-1006. doi: 10.1097/ALN.0000000000005153. Anesthesiology. 2024. PMID: 39240535 Review.
-
Imbalance in Unc80 RNA Editing Disrupts Dynamic Neuronal Activity and Olfactory Perception.Int J Mol Sci. 2024 May 30;25(11):5985. doi: 10.3390/ijms25115985. Int J Mol Sci. 2024. PMID: 38892173 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous


