Coordination of Fc receptor signaling regulates cellular commitment to phagocytosis
- PMID: 20974965
- PMCID: PMC2984174
- DOI: 10.1073/pnas.1008248107
Coordination of Fc receptor signaling regulates cellular commitment to phagocytosis
Abstract
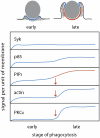
During Fcγ receptor (FcR)-mediated phagocytosis by macrophages, cytoplasm advances over IgG-coated particles by the sequential ligation of FcR in plasma membranes. If FcR signaling was strictly autonomous, then the signals generated during phagocytosis should be proportional to the number of ligated receptors. By measuring FcR-dependent responses to beads coated with various densities of IgG, this study identified nonlinear signaling that organizes an all or none response during particle ingestion. Phagocytosis of beads with IgG at low density either stalled after making small, actin-rich cups or proceeded to completion at the same rate as phagocytosis of high-density IgG beads. Signals were measured by quantifying the recruitment of YFP-labeled probes to phagocytic cup membranes. Although the magnitude of early signals correlated with IgG density, later signals showed an all or none response, which was regulated by the concentrations of 3' phosphoinositides in phagocytic cup membranes. Thus, 3' phosphoinositides, shown previously to be required for phagocytosis, function in a feedback regulatory mechanism affecting late but not early signals. This indicates a mechanism for the coordination of cell movements initiated by receptor signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

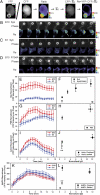
 (a single number determined for each cell) generated a YFP path-length image representing how that cell's quantity of YFP chimera would appear if it did not contain any localization information. Subtracting this image from the YFP image created a difference image, Re, which represented the quantity of YFP displaced by localization information. (B–D) Phase contrast and Re images of Syk-YFP–expressing RAWs internalizing B10 (B) and B1 (C) and Syk(R194A)-YFP–expressing RAW 264.7 macrophages internalizing B10 (D). Arrowheads indicate regions with increased recruitment of Syk-YFP. (Scale bar: 5 μm.) The average recruitments of Syk-YFP (E), YFP-p85 (G), YFP-actin (I), and YFP-AktPH (K) per pixel in phagosomes of B10 (red) and B1 (blue) were plotted as a function of time, aligned by the start of membrane movement for phagocytosis. (F) The maximum Syk-YFP recruitment averaged from single phagocytic events for B10, B6, B3, and B1 (solid circles; n = 8) as well as Syk(R194A)-YFP recruitment for B10 and B1 (open triangles; n = 4). B10 phagosomes recruited more YFP-p85 (H); *P = 0.024) and YFP-actin (J; *P = 0.029) than B1 phagosomes. Stalled phagocytic cups recruited slightly less YFP-actin than did completed phagosomes (open circles; n = 5; **P = 0.129). (L) The maximum YFP-AktPH recruitment during completed phagocytic events (solid circles; n = 8) showed no difference between B10 and B1 (*P = 0.223). In contrast, YFP-AktPH recruitment in stalled phagocytic cups (open circle; n = 5) increased slightly with ligand density but remained significantly lower than in finished phagosomes (**P = 0.001).
(a single number determined for each cell) generated a YFP path-length image representing how that cell's quantity of YFP chimera would appear if it did not contain any localization information. Subtracting this image from the YFP image created a difference image, Re, which represented the quantity of YFP displaced by localization information. (B–D) Phase contrast and Re images of Syk-YFP–expressing RAWs internalizing B10 (B) and B1 (C) and Syk(R194A)-YFP–expressing RAW 264.7 macrophages internalizing B10 (D). Arrowheads indicate regions with increased recruitment of Syk-YFP. (Scale bar: 5 μm.) The average recruitments of Syk-YFP (E), YFP-p85 (G), YFP-actin (I), and YFP-AktPH (K) per pixel in phagosomes of B10 (red) and B1 (blue) were plotted as a function of time, aligned by the start of membrane movement for phagocytosis. (F) The maximum Syk-YFP recruitment averaged from single phagocytic events for B10, B6, B3, and B1 (solid circles; n = 8) as well as Syk(R194A)-YFP recruitment for B10 and B1 (open triangles; n = 4). B10 phagosomes recruited more YFP-p85 (H); *P = 0.024) and YFP-actin (J; *P = 0.029) than B1 phagosomes. Stalled phagocytic cups recruited slightly less YFP-actin than did completed phagosomes (open circles; n = 5; **P = 0.129). (L) The maximum YFP-AktPH recruitment during completed phagocytic events (solid circles; n = 8) showed no difference between B10 and B1 (*P = 0.223). In contrast, YFP-AktPH recruitment in stalled phagocytic cups (open circle; n = 5) increased slightly with ligand density but remained significantly lower than in finished phagosomes (**P = 0.001).
Similar articles
-
The coordination of signaling during Fc receptor-mediated phagocytosis.J Leukoc Biol. 2004 Dec;76(6):1093-103. doi: 10.1189/jlb.0804439. Epub 2004 Oct 5. J Leukoc Biol. 2004. PMID: 15466916 Review.
-
Cofilin contributes to phagocytosis of IgG-opsonized particles but not non-opsonized particles in RAW264 macrophages.Microscopy (Oxf). 2016 Jun;65(3):233-42. doi: 10.1093/jmicro/dfv376. Epub 2016 Jan 10. Microscopy (Oxf). 2016. PMID: 26754560
-
Antibody-mediated erythrolysis and erythrophagocytosis by human monocytes, macrophages and activated macrophages. Evidence for distinction between involvement of high-affinity and low-affinity receptors for IgG by using different erythroid target cells.Immunology. 1988 Mar;63(3):513-20. Immunology. 1988. PMID: 2965100 Free PMC article.
-
Tight nanoscale clustering of Fcγ receptors using DNA origami promotes phagocytosis.Elife. 2021 Jun 3;10:e68311. doi: 10.7554/eLife.68311. Elife. 2021. PMID: 34080973 Free PMC article.
-
Signal transduction during Fc receptor-mediated phagocytosis.J Leukoc Biol. 2002 Dec;72(6):1092-108. J Leukoc Biol. 2002. PMID: 12488490 Review.
Cited by
-
Amyloid-β in Alzheimer's disease: Structure, toxicity, distribution, treatment, and prospects.Ibrain. 2024 May 23;10(3):266-289. doi: 10.1002/ibra.12155. eCollection 2024 Fall. Ibrain. 2024. PMID: 39346788 Free PMC article. Review.
-
Prior Fc receptor activation primes macrophages for increased sensitivity to IgG via long-term and short-term mechanisms.Dev Cell. 2024 Nov 4;59(21):2882-2896.e7. doi: 10.1016/j.devcel.2024.07.017. Epub 2024 Aug 12. Dev Cell. 2024. PMID: 39137774
-
The multivalency game ruling the biology of immunity.Biophys Rev (Melville). 2023 Dec 29;4(4):041306. doi: 10.1063/5.0166165. eCollection 2023 Dec. Biophys Rev (Melville). 2023. PMID: 38505426 Free PMC article. Review.
-
Antibody surface mobility amplifies FcγR signaling via Arp2/3 during phagocytosis.Biophys J. 2024 Aug 6;123(15):2312-2327. doi: 10.1016/j.bpj.2024.01.036. Epub 2024 Feb 5. Biophys J. 2024. PMID: 38321740
-
DNA origami: Interrogating the nano-landscape of immune receptor activation.Biophys J. 2024 Aug 6;123(15):2211-2223. doi: 10.1016/j.bpj.2023.10.013. Epub 2023 Oct 14. Biophys J. 2024. PMID: 37838832 Review.
References
-
- Swanson JA, Hoppe AD. The coordination of signaling during Fc receptor-mediated phagocytosis. J Leukoc Biol. 2004;76:1093–1103. - PubMed
-
- Nimmerjahn F, Ravetch JV. Fcgamma receptors: Old friends and new family members. Immunity. 2006;24:19–28. - PubMed
-
- May RC, Machesky LM. Phagocytosis and the actin cytoskeleton. J Cell Sci. 2001;114:1061–1077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


