HIGS: host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis
- PMID: 20884801
- PMCID: PMC2965548
- DOI: 10.1105/tpc.110.077040
HIGS: host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis
Abstract
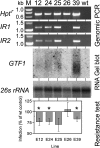
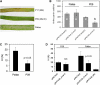
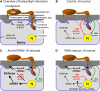
Powdery mildew fungi are obligate biotrophic pathogens that only grow on living hosts and cause damage in thousands of plant species. Despite their agronomical importance, little direct functional evidence for genes of pathogenicity and virulence is currently available because mutagenesis and transformation protocols are lacking. Here, we show that the accumulation in barley (Hordeum vulgare) and wheat (Triticum aestivum) of double-stranded or antisense RNA targeting fungal transcripts affects the development of the powdery mildew fungus Blumeria graminis. Proof of concept for host-induced gene silencing was obtained by silencing the effector gene Avra10, which resulted in reduced fungal development in the absence, but not in the presence, of the matching resistance gene Mla10. The fungus could be rescued from the silencing of Avra10 by the transient expression of a synthetic gene that was resistant to RNA interference (RNAi) due to silent point mutations. The results suggest traffic of RNA molecules from host plants into B. graminis and may lead to an RNAi-based crop protection strategy against fungal pathogens.
Figures





Similar articles
-
Small RNAs from cereal powdery mildew pathogens may target host plant genes.Fungal Biol. 2018 Nov;122(11):1050-1063. doi: 10.1016/j.funbio.2018.08.008. Epub 2018 Sep 11. Fungal Biol. 2018. PMID: 30342621
-
Small RNA discovery in the interaction between barley and the powdery mildew pathogen.BMC Genomics. 2019 Jul 25;20(1):610. doi: 10.1186/s12864-019-5947-z. BMC Genomics. 2019. PMID: 31345162 Free PMC article.
-
Interaction of a Blumeria graminis f. sp. hordei effector candidate with a barley ARF-GAP suggests that host vesicle trafficking is a fungal pathogenicity target.Mol Plant Pathol. 2014 Aug;15(6):535-49. doi: 10.1111/mpp.12110. Epub 2014 Mar 3. Mol Plant Pathol. 2014. PMID: 24304971 Free PMC article.
-
Cereal powdery mildew effectors: a complex toolbox for an obligate pathogen.Curr Opin Microbiol. 2018 Dec;46:26-33. doi: 10.1016/j.mib.2018.01.018. Epub 2018 Feb 22. Curr Opin Microbiol. 2018. PMID: 29455142 Review.
-
RNAi Technology: A New Path for the Research and Management of Obligate Biotrophic Phytopathogenic Fungi.Int J Mol Sci. 2023 May 22;24(10):9082. doi: 10.3390/ijms24109082. Int J Mol Sci. 2023. PMID: 37240427 Free PMC article. Review.
Cited by
-
Transformation-based gene silencing and functional characterization of an ISC effector reveal how a powdery mildew fungus disturbs salicylic acid biosynthesis and immune response in the plant.Mol Plant Pathol. 2024 Nov;25(11):e70030. doi: 10.1111/mpp.70030. Mol Plant Pathol. 2024. PMID: 39558488 Free PMC article.
-
RNAi-Based Approaches to Control Mycotoxin Producers: Challenges and Perspectives.J Fungi (Basel). 2024 Sep 29;10(10):682. doi: 10.3390/jof10100682. J Fungi (Basel). 2024. PMID: 39452634 Free PMC article. Review.
-
Harnessing RNA interference for the control of Fusarium species: A critical review.Mol Plant Pathol. 2024 Oct;25(10):e70011. doi: 10.1111/mpp.70011. Mol Plant Pathol. 2024. PMID: 39363756 Free PMC article. Review.
-
Mycologists and Virologists Align: Proposing Botrytis cinerea for Global Mycovirus Studies.Viruses. 2024 Sep 18;16(9):1483. doi: 10.3390/v16091483. Viruses. 2024. PMID: 39339959 Free PMC article. Review.
-
Plant-induced bacterial gene silencing: a novel control method for bacterial wilt disease.Front Plant Sci. 2024 Aug 2;15:1411837. doi: 10.3389/fpls.2024.1411837. eCollection 2024. Front Plant Sci. 2024. PMID: 39157516 Free PMC article.
References
-
- An Q.L., Ehlers K., Kogel K.H., van Bel A.J.E., Hückelhoven R. (2006). Multivesicular compartments proliferate in susceptible and resistant MLA12-barley leaves in response to infection by the biotrophic powdery mildew fungus. New Phytol. 172: 563–576 - PubMed
-
- Baum J.A., Bogaert T., Clinton W., Heck G.R., Feldmann P., Ilagan O., Johnson S., Plaetinck G., Munyikwa T., Pleau M., Vaughn T., Roberts J. (2007). Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 25: 1322–1326 - PubMed
-
- Both M., Eckert S.E., Csukai M., Müller E., Dimopoulos G., Spanu P.D. (2005a). Transcript profiles of Blumeria graminis development during infection reveal a cluster of genes that are potential virulence determinants. Mol. Plant Microbe Interact. 18: 125–133 - PubMed
-
- Bruun-Rasmussen M., Madsen C.T., Jessing S., Albrechtsen M. (2007). Stability of Barley stripe mosaic virus-induced gene silencing in barley. Mol. Plant Microbe Interact. 20: 1323–1331 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


