Salmonid T cells assemble in the thymus, spleen and in novel interbranchial lymphoid tissue
- PMID: 20880086
- PMCID: PMC3039185
- DOI: 10.1111/j.1469-7580.2010.01305.x
Salmonid T cells assemble in the thymus, spleen and in novel interbranchial lymphoid tissue
Abstract
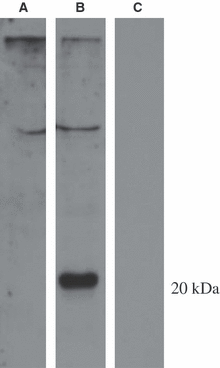
In modern bony fishes, or teleost fish, the general lack of leucocyte markers has greatly hampered investigations of the anatomy of the immune system and its reactions involved in inflammatory responses. We have previously reported the cloning and sequencing of the salmon CD3 complex, molecules that are specifically expressed in T cells. Here, we generate and validate sera recognizing a peptide sequence of the CD3ε chain. Flow cytometry analysis revealed high numbers of CD3ε(+) or T cells in the thymus, gill and intestine, whereas lower numbers were detected in the head kidney, spleen and peripheral blood leucocytes. Subsequent morphological analysis showed accumulations of T cells in the thymus and spleen and in the newly discovered gill-located interbranchial lymphoid tissue. In the latter, the T cells are embedded in a meshwork of epithelial cells and in the spleen, they cluster in the white pulp surrounding ellipsoids. The anatomical organization of the salmonid thymic cortex and medulla seems to be composed of three layers consisting of a sub-epithelial medulla-like zone, an intermediate cortex-like zone and finally another cortex-like basal zone. Our study in the salmonid thymus reports a previously non-described tissue organization. In the intestinal tract, abundant T cells were found embedded in the epithelium. In non-lymphoid organs, the presence of T cells was limited. The results show that the interbranchial lymphoid tissue is quantitatively a very important site of T cell aggregation, strategically located to facilitate antigen encounter. The interbranchial lymphoid tissue has no resemblance to previously described lymphoid tissues.
© 2010 The Authors. Journal of Anatomy © 2010 Anatomical Society of Great Britain and Ireland.
Figures




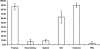


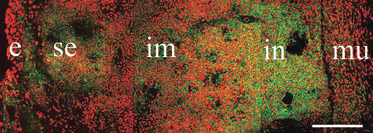

Similar articles
-
Anatomy of teleost fish immune structures and organs.Immunogenetics. 2021 Feb;73(1):53-63. doi: 10.1007/s00251-020-01196-0. Epub 2021 Jan 11. Immunogenetics. 2021. PMID: 33426583 Free PMC article. Review.
-
Transcriptional characterization of the T cell population within the salmonid interbranchial lymphoid tissue.J Immunol. 2014 Oct 1;193(7):3463-9. doi: 10.4049/jimmunol.1400797. Epub 2014 Aug 29. J Immunol. 2014. PMID: 25172486
-
Ontogeny of IgM-producing cells in the mandarin fish Siniperca chuatsi identified by in situ hybridisation.Vet Immunol Immunopathol. 2009 Dec 15;132(2-4):146-52. doi: 10.1016/j.vetimm.2009.05.018. Epub 2009 Jun 6. Vet Immunol Immunopathol. 2009. PMID: 19556013
-
The interbranchial lymphoid tissue of Atlantic Salmon (Salmo salar L) extends as a diffuse mucosal lymphoid tissue throughout the trailing edge of the gill filament.J Morphol. 2015 Sep;276(9):1075-88. doi: 10.1002/jmor.20403. Epub 2015 May 26. J Morphol. 2015. PMID: 26011185
-
Histological development of the thymic and intestinal lymphoid tissue of the horse.J S Afr Vet Assoc. 1975 Mar;46(1):47-55. J S Afr Vet Assoc. 1975. PMID: 1100827 Review.
Cited by
-
Dynamics of Gill Responses to a Natural Infection with Neoparamoeba perurans in Farmed Tasmanian Atlantic Salmon.Animals (Basel). 2024 Aug 15;14(16):2356. doi: 10.3390/ani14162356. Animals (Basel). 2024. PMID: 39199891 Free PMC article.
-
Resistance of Nile tilapia fed with Padina boergesenii extract to Pseudomonas putida infection.BMC Vet Res. 2024 Jun 29;20(1):281. doi: 10.1186/s12917-024-04115-7. BMC Vet Res. 2024. PMID: 38951863 Free PMC article.
-
The thymus and T-cell ontogeny in ballan wrasse (Labrus bergylta) is nutritionally modelled.Front Immunol. 2023 May 1;14:1166785. doi: 10.3389/fimmu.2023.1166785. eCollection 2023. Front Immunol. 2023. PMID: 37197651 Free PMC article.
-
Cluster of differentiation antigens: essential roles in the identification of teleost fish T lymphocytes.Mar Life Sci Technol. 2022 Aug 19;4(3):303-316. doi: 10.1007/s42995-022-00136-z. eCollection 2022 Aug. Mar Life Sci Technol. 2022. PMID: 37073166 Free PMC article. Review.
-
Immunohistochemistry of the Gut-Associated Lymphoid Tissue (GALT) in African Bonytongue (Heterotis niloticus, Cuvier 1829).Int J Mol Sci. 2023 Jan 24;24(3):2316. doi: 10.3390/ijms24032316. Int J Mol Sci. 2023. PMID: 36768639 Free PMC article.
References
-
- Alder MN, Herrin BR, Sadlonova A, et al. Antibody responses of variable lymphocyte responses in the lamprey. Nat Immunol. 2008;9:319–327. - PubMed
-
- Boehm T, Bleul CC. The evolutionary history of lymphoid organs. Nat Immunol. 2007;8:131–135. - PubMed
-
- Bowden TJ, Cook P, Rombout JH. Development and function of the thymus in teleosts. Fish Shellfish Immunol. 2005;19:413–427. - PubMed
-
- Brandtzaeg P, Kiyono H, Pabst R, et al. Terminology: nomenclature of the mucosa-associated lymphoid tissue. Mucosal Immunol. 2008;1:31–37. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources


