RETRACTED: Human SIRT6 promotes DNA end resection through CtIP deacetylation
- PMID: 20829486
- PMCID: PMC3276839
- DOI: 10.1126/science.1192049
RETRACTED: Human SIRT6 promotes DNA end resection through CtIP deacetylation
Retraction in
-
Retraction.Science. 2019 Apr 19;364(6437):247. doi: 10.1126/science.aax4558. Epub 2019 Apr 11. Science. 2019. PMID: 30975768 Free PMC article. No abstract available.
Expression of concern in
-
Editorial expression of concern.Science. 2018 Sep 28;361(6409):1322. doi: 10.1126/science.aav4528. Epub 2018 Sep 27. Science. 2018. PMID: 30262489 No abstract available.
Abstract
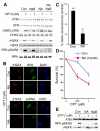
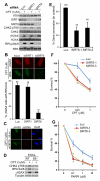
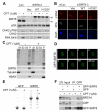
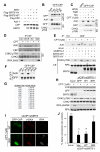
SIRT6 belongs to the sirtuin family of protein lysine deacetylases, which regulate aging and genome stability. We found that human SIRT6 has a role in promoting DNA end resection, a crucial step in DNA double-strand break (DSB) repair by homologous recombination. SIRT6 depletion impaired the accumulation of replication protein A and single-stranded DNA at DNA damage sites, reduced rates of homologous recombination, and sensitized cells to DSB-inducing agents. We identified the DSB resection protein CtIP [C-terminal binding protein (CtBP) interacting protein] as a SIRT6 interaction partner and showed that SIRT6-dependent CtIP deacetylation promotes resection. A nonacetylatable CtIP mutant alleviated the effect of SIRT6 depletion on resection, thus identifying CtIP as a key substrate by which SIRT6 facilitates DSB processing and homologous recombination. These findings further clarify how SIRT6 promotes genome stability.
Figures
Similar articles
-
Human CtIP promotes DNA end resection.Nature. 2007 Nov 22;450(7169):509-14. doi: 10.1038/nature06337. Epub 2007 Oct 28. Nature. 2007. PMID: 17965729 Free PMC article.
-
DNA end resection by CtIP and exonuclease 1 prevents genomic instability.EMBO Rep. 2010 Dec;11(12):962-8. doi: 10.1038/embor.2010.157. Epub 2010 Nov 5. EMBO Rep. 2010. PMID: 21052091 Free PMC article.
-
The interaction of CtIP and Nbs1 connects CDK and ATM to regulate HR-mediated double-strand break repair.PLoS Genet. 2013;9(2):e1003277. doi: 10.1371/journal.pgen.1003277. Epub 2013 Feb 28. PLoS Genet. 2013. PMID: 23468639 Free PMC article.
-
CtIP: A DNA damage response protein at the intersection of DNA metabolism.DNA Repair (Amst). 2015 Aug;32:75-81. doi: 10.1016/j.dnarep.2015.04.016. Epub 2015 May 2. DNA Repair (Amst). 2015. PMID: 25957490 Review.
-
DNA damage and decisions: CtIP coordinates DNA repair and cell cycle checkpoints.Trends Cell Biol. 2010 Jul;20(7):402-9. doi: 10.1016/j.tcb.2010.04.002. Epub 2010 May 3. Trends Cell Biol. 2010. PMID: 20444606 Free PMC article. Review.
Cited by
-
Recent trends: Retractions of articles in the oncology field.Heliyon. 2024 Jun 14;10(12):e33007. doi: 10.1016/j.heliyon.2024.e33007. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38984306 Free PMC article.
-
The dual role of sirtuins in cancer: biological functions and implications.Front Oncol. 2024 Jun 14;14:1384928. doi: 10.3389/fonc.2024.1384928. eCollection 2024. Front Oncol. 2024. PMID: 38947884 Free PMC article. Review.
-
Impact of NAD+ metabolism on ovarian aging.Immun Ageing. 2023 Dec 2;20(1):70. doi: 10.1186/s12979-023-00398-w. Immun Ageing. 2023. PMID: 38041117 Free PMC article. Review.
-
SIRT6's function in controlling the metabolism of lipids and glucose in diabetic nephropathy.Front Endocrinol (Lausanne). 2023 Oct 9;14:1244705. doi: 10.3389/fendo.2023.1244705. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37876546 Free PMC article. Review.
-
Emerging Roles of SIRT5 in Metabolism, Cancer, and SARS-CoV-2 Infection.Cells. 2023 Mar 9;12(6):852. doi: 10.3390/cells12060852. Cells. 2023. PMID: 36980194 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases


