Competing streams at the cocktail party: exploring the mechanisms of attention and temporal integration
- PMID: 20826671
- PMCID: PMC2942024
- DOI: 10.1523/JNEUROSCI.0827-10.2010
Competing streams at the cocktail party: exploring the mechanisms of attention and temporal integration
Abstract
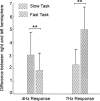
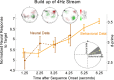
Processing of complex acoustic scenes depends critically on the temporal integration of sensory information as sounds evolve naturally over time. It has been previously speculated that this process is guided by both innate mechanisms of temporal processing in the auditory system, as well as top-down mechanisms of attention and possibly other schema-based processes. In an effort to unravel the neural underpinnings of these processes and their role in scene analysis, we combine magnetoencephalography (MEG) with behavioral measures in humans in the context of polyrhythmic tone sequences. While maintaining unchanged sensory input, we manipulate subjects' attention to one of two competing rhythmic streams in the same sequence. The results reveal that the neural representation of the attended rhythm is significantly enhanced in both its steady-state power and spatial phase coherence relative to its unattended state, closely correlating with its perceptual detectability for each listener. Interestingly, the data reveal a differential efficiency of rhythmic rates of the order of few hertz during the streaming process, closely following known neural and behavioral measures of temporal modulation sensitivity in the auditory system. These findings establish a direct link between known temporal modulation tuning in the auditory system (particularly at the level of auditory cortex) and the temporal integration of perceptual features in a complex acoustic scene, while mediated by processes of attention.
Figures




Similar articles
-
Decoding Object-Based Auditory Attention from Source-Reconstructed MEG Alpha Oscillations.J Neurosci. 2021 Oct 13;41(41):8603-8617. doi: 10.1523/JNEUROSCI.0583-21.2021. Epub 2021 Aug 24. J Neurosci. 2021. PMID: 34429378 Free PMC article.
-
Perceptual organization of auditory streaming-task relies on neural entrainment of the stimulus-presentation rate: MEG evidence.BMC Neurosci. 2013 Oct 12;14:120. doi: 10.1186/1471-2202-14-120. BMC Neurosci. 2013. PMID: 24119225 Free PMC article.
-
Where is the cocktail party? Decoding locations of attended and unattended moving sound sources using EEG.Neuroimage. 2020 Jan 15;205:116283. doi: 10.1016/j.neuroimage.2019.116283. Epub 2019 Oct 17. Neuroimage. 2020. PMID: 31629828
-
The role of auditory cortex in the formation of auditory streams.Hear Res. 2007 Jul;229(1-2):116-31. doi: 10.1016/j.heares.2007.01.007. Epub 2007 Jan 16. Hear Res. 2007. PMID: 17307315 Free PMC article. Review.
-
The encoding of auditory objects in auditory cortex: insights from magnetoencephalography.Int J Psychophysiol. 2015 Feb;95(2):184-90. doi: 10.1016/j.ijpsycho.2014.05.005. Epub 2014 May 16. Int J Psychophysiol. 2015. PMID: 24841996 Free PMC article. Review.
Cited by
-
Neural dynamics underlying successful auditory short-term memory performance.Eur J Neurosci. 2023 Oct;58(8):3859-3878. doi: 10.1111/ejn.16140. Epub 2023 Sep 10. Eur J Neurosci. 2023. PMID: 37691137 Free PMC article.
-
Investigating the influence of masker and target properties on the dynamics of perceptual awareness under informational masking.PLoS One. 2023 Mar 16;18(3):e0282885. doi: 10.1371/journal.pone.0282885. eCollection 2023. PLoS One. 2023. PMID: 36928693 Free PMC article.
-
Modeling the Repetition-Based Recovering of Acoustic and Visual Sources With Dendritic Neurons.Front Neurosci. 2022 Apr 28;16:855753. doi: 10.3389/fnins.2022.855753. eCollection 2022. Front Neurosci. 2022. PMID: 35573290 Free PMC article.
-
Making sense of periodicity glimpses in a prediction-update-loop-A computational model of attentive voice tracking.J Acoust Soc Am. 2022 Feb;151(2):712. doi: 10.1121/10.0009337. J Acoust Soc Am. 2022. PMID: 35232067 Free PMC article.
-
The Brain Tracks Multiple Predictions About the Auditory Scene.Front Hum Neurosci. 2021 Nov 3;15:747769. doi: 10.3389/fnhum.2021.747769. eCollection 2021. Front Hum Neurosci. 2021. PMID: 34803633 Free PMC article.
References
-
- Ahmar NE, Simon JZ. MEG adaptive noise suppression using fast LMS. Presented at the Second International IEEE Engineering in Medicine and Biology Society Conference on Neural Engineering; March 16–19; Washington, DC. 2005.
-
- Ahmar NE, Wang Y, Simon JZ. Significance tests for MEG response detection. Presented at the Second International IEEE Engineering in Medicine and Biology Society Conference on Neural Engineering; March 16–19; Washington, DC. 2005.
-
- Alain C. Breaking the wave: effects of attention and learning on concurrent sound perception. Hear Res. 2007;229:225–236. - PubMed
-
- Alcaini M, Giard MH, Echallier JF, Pernier J. Selective auditory attention effects in tonotopically organized cortical areas: a topographic ERP study. Hum Brain Mapp. 1995;2:59–169.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

