Production of glycoprotein vaccines in Escherichia coli
- PMID: 20701771
- PMCID: PMC2927510
- DOI: 10.1186/1475-2859-9-61
Production of glycoprotein vaccines in Escherichia coli
Abstract
Background: Conjugate vaccines in which polysaccharide antigens are covalently linked to carrier proteins belong to the most effective and safest vaccines against bacterial pathogens. State-of-the art production of conjugate vaccines using chemical methods is a laborious, multi-step process. In vivo enzymatic coupling using the general glycosylation pathway of Campylobacter jejuni in recombinant Escherichia coli has been suggested as a simpler method for producing conjugate vaccines. In this study we describe the in vivo biosynthesis of two novel conjugate vaccine candidates against Shigella dysenteriae type 1, an important bacterial pathogen causing severe gastro-intestinal disease states mainly in developing countries.
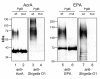
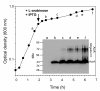
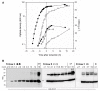
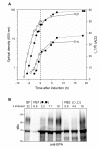
Results: Two different periplasmic carrier proteins, AcrA from C. jejuni and a toxoid form of Pseudomonas aeruginosa exotoxin were glycosylated with Shigella O antigens in E. coli. Starting from shake flask cultivation in standard complex medium a lab-scale fed-batch process was developed for glycoconjugate production. It was found that efficiency of glycosylation but not carrier protein expression was highly susceptible to the physiological state at induction. After induction glycoconjugates generally appeared later than unglycosylated carrier protein, suggesting that glycosylation was the rate-limiting step for synthesis of conjugate vaccines in E. coli. Glycoconjugate synthesis, in particular expression of oligosaccharyltransferase PglB, strongly inhibited growth of E. coli cells after induction, making it necessary to separate biomass growth and recombinant protein expression phases. With a simple pulse and linear feed strategy and the use of semi-defined glycerol medium, volumetric glycoconjugate yield was increased 30 to 50-fold.
Conclusions: The presented data demonstrate that glycosylated proteins can be produced in recombinant E. coli at a larger scale. The described methodologies constitute an important step towards cost-effective in vivo production of conjugate vaccines, which in future may be used for combating severe infectious diseases, particularly in developing countries.
Figures


Similar articles
-
In vivo production of a novel glycoconjugate vaccine against Shigella flexneri 2a in recombinant Escherichia coli: identification of stimulating factors for in vivo glycosylation.Microb Cell Fact. 2015 Jan 23;14:12. doi: 10.1186/s12934-015-0195-7. Microb Cell Fact. 2015. PMID: 25612741 Free PMC article.
-
Purification and characterization of a Shigella conjugate vaccine, produced by glycoengineering Escherichia coli.Glycobiology. 2016 Jan;26(1):51-62. doi: 10.1093/glycob/cwv077. Epub 2015 Sep 9. Glycobiology. 2016. PMID: 26353918
-
Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli.Proc Natl Acad Sci U S A. 2005 Feb 22;102(8):3016-21. doi: 10.1073/pnas.0500044102. Epub 2005 Feb 9. Proc Natl Acad Sci U S A. 2005. PMID: 15703289 Free PMC article.
-
Hijacking bacterial glycosylation for the production of glycoconjugates, from vaccines to humanised glycoproteins.J Pharm Pharmacol. 2015 Mar;67(3):338-50. doi: 10.1111/jphp.12321. Epub 2014 Sep 22. J Pharm Pharmacol. 2015. PMID: 25244672 Free PMC article. Review.
-
Advances in Bacterial Oligosaccharyltransferase Structure Elucidation and Potential Application to Glycoconjugate Vaccine Design.Front Biosci (Landmark Ed). 2023 Nov 28;28(11):305. doi: 10.31083/j.fbl2811305. Front Biosci (Landmark Ed). 2023. PMID: 38062836 Review.
Cited by
-
Beyond Antibiotics: What the Future Holds.Antibiotics (Basel). 2024 Sep 25;13(10):919. doi: 10.3390/antibiotics13100919. Antibiotics (Basel). 2024. PMID: 39452186 Free PMC article. Review.
-
Immunogenicity of Current and Next-Generation Pneumococcal Conjugate Vaccines in Children: Current Challenges and Upcoming Opportunities.Open Forum Infect Dis. 2024 May 6;11(5):ofae220. doi: 10.1093/ofid/ofae220. eCollection 2024 May. Open Forum Infect Dis. 2024. PMID: 38770212 Free PMC article. Review.
-
Development of a novel glycoengineering platform for the rapid production of conjugate vaccines.Microb Cell Fact. 2023 Aug 18;22(1):159. doi: 10.1186/s12934-023-02125-y. Microb Cell Fact. 2023. PMID: 37596672 Free PMC article.
-
Immunogenicity and protective efficacy of a prototype pneumococcal bioconjugate vaccine.Vaccine. 2022 Oct 6;40(42):6107-6113. doi: 10.1016/j.vaccine.2022.09.018. Epub 2022 Sep 15. Vaccine. 2022. PMID: 36115800 Free PMC article.
-
Immunoinformatic analysis of the whole proteome for vaccine design: An application to Clostridium perfringens.Front Immunol. 2022 Aug 30;13:942907. doi: 10.3389/fimmu.2022.942907. eCollection 2022. Front Immunol. 2022. PMID: 36110855 Free PMC article.
References
-
- Taylor DN, Trofa AC, Sadoff J, Chu CY, Bryla D, Shiloach J, Cohen D, Ashkenazi S, Lerman Y, Egan W, Schneerson R, Robbins JB. Synthesis, characterization, and clinical evaluation of conjugate vaccines composed of the O-specific polysaccharides of Shigella dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei (Plesiomonas shigelloides) bound to bacterial toxoids. Infect Immun. 1993;61(9):3678–3687. - PMC - PubMed
-
- Robbins JB, Schneerson R, Szu SC, Pozsgay V. Bacterial polysaccharide-protein conjugate vaccines. Pure Appl Chem. 1999;71(5):745–754. doi: 10.1351/pac199971050745. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous


