TAL nucleases (TALNs): hybrid proteins composed of TAL effectors and FokI DNA-cleavage domain
- PMID: 20699274
- PMCID: PMC3017587
- DOI: 10.1093/nar/gkq704
TAL nucleases (TALNs): hybrid proteins composed of TAL effectors and FokI DNA-cleavage domain
Abstract
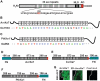
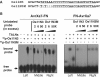
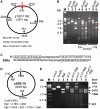
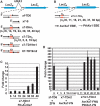
DNA double-strand breaks enhance homologous recombination in cells and have been exploited for targeted genome editing through use of engineered endonucleases. Here we report the creation and initial characterization of a group of rare-cutting, site-specific DNA nucleases produced by fusion of the restriction enzyme FokI endonuclease domain (FN) with the high-specificity DNA-binding domains of AvrXa7 and PthXo1. AvrXa7 and PthXo1 are members of the transcription activator-like (TAL) effector family whose central repeat units dictate target DNA recognition and can be modularly constructed to create novel DNA specificity. The hybrid FN-AvrXa7, AvrXa7-FN and PthXo1-FN proteins retain both recognition specificity for their target DNA (a 26 bp sequence for AvrXa7 and 24 bp for PthXo1) and the double-stranded DNA cleaving activity of FokI and, thus, are called TAL nucleases (TALNs). With all three TALNs, DNA is cleaved adjacent to the TAL-binding site under optimal conditions in vitro. When expressed in yeast, the TALNs promote DNA homologous recombination of a LacZ gene containing paired AvrXa7 or asymmetric AvrXa7/PthXo1 target sequences. Our results demonstrate the feasibility of creating a tool box of novel TALNs with potential for targeted genome modification in organisms lacking facile mechanisms for targeted gene knockout and homologous recombination.
Figures

Similar articles
-
Targeting DNA double-strand breaks with TAL effector nucleases.Genetics. 2010 Oct;186(2):757-61. doi: 10.1534/genetics.110.120717. Epub 2010 Jul 26. Genetics. 2010. PMID: 20660643 Free PMC article.
-
The virulence factor AvrXa7 of Xanthomonas oryzae pv. oryzae is a type III secretion pathway-dependent nuclear-localized double-stranded DNA-binding protein.Proc Natl Acad Sci U S A. 2000 Aug 15;97(17):9807-12. doi: 10.1073/pnas.170286897. Proc Natl Acad Sci U S A. 2000. PMID: 10931960 Free PMC article.
-
Modularly assembled designer TAL effector nucleases for targeted gene knockout and gene replacement in eukaryotes.Nucleic Acids Res. 2011 Aug;39(14):6315-25. doi: 10.1093/nar/gkr188. Epub 2011 Mar 31. Nucleic Acids Res. 2011. PMID: 21459844 Free PMC article.
-
Genome engineering with TAL-effector nucleases and alternative modular nuclease technologies.Curr Gene Ther. 2013 Aug;13(4):291-303. doi: 10.2174/15665232113139990026. Curr Gene Ther. 2013. PMID: 23888878 Review.
-
Origins of Programmable Nucleases for Genome Engineering.J Mol Biol. 2016 Feb 27;428(5 Pt B):963-89. doi: 10.1016/j.jmb.2015.10.014. Epub 2015 Oct 23. J Mol Biol. 2016. PMID: 26506267 Free PMC article. Review.
Cited by
-
Fusion of FokI and catalytically inactive prokaryotic Argonautes enables site-specific programmable DNA cleavage.J Biol Chem. 2024 Sep;300(9):107720. doi: 10.1016/j.jbc.2024.107720. Epub 2024 Aug 28. J Biol Chem. 2024. PMID: 39214308 Free PMC article.
-
Designer TALEs enable discovery of cell death-inducer genes.Plant Physiol. 2024 Jul 31;195(4):2985-2996. doi: 10.1093/plphys/kiae230. Plant Physiol. 2024. PMID: 38723194 Free PMC article.
-
The pathogenic mechanism of syndactyly type V identified in a Hoxd13Q50R knock-in mice.Bone Res. 2024 Apr 1;12(1):21. doi: 10.1038/s41413-024-00322-y. Bone Res. 2024. PMID: 38561387 Free PMC article.
-
Autologous gene therapy for hemoglobinopathies: From bench to patient's bedside.Mol Ther. 2024 May 1;32(5):1202-1218. doi: 10.1016/j.ymthe.2024.03.005. Epub 2024 Mar 7. Mol Ther. 2024. PMID: 38454604 Review.
-
Enhancement of specialized metabolites using CRISPR/Cas gene editing technology in medicinal plants.Front Plant Sci. 2024 Feb 21;15:1279738. doi: 10.3389/fpls.2024.1279738. eCollection 2024. Front Plant Sci. 2024. PMID: 38450402 Free PMC article. Review.
References
-
- Le Provost F, Lillico S, Passet B, Young R, Whitelaw B, Vilotte JL. Zinc finger nuclease technology heralds a new era in mammalian transgenesis. Trends Biotechnol. 2010;28:134–141. - PubMed
-
- Jasin M. Genetic manipulation of genomes with rare-cutting endonucleases. Trends Genet. 1996;12:224–228. - PubMed
-
- Bibikova M, Beumer K, Trautman JK, Carroll D. Enhancing gene targeting with designed zinc finger nucleases. Science. 2003;300:764. - PubMed
-
- Porteus MH, Carroll D. Gene targeting using zinc finger nucleases. Nat. Biotechnol. 2005;23:967–973. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous


