Susceptibility pathways in Fanconi's anemia and breast cancer
- PMID: 20484397
- PMCID: PMC3069698
- DOI: 10.1056/NEJMra0809889
Susceptibility pathways in Fanconi's anemia and breast cancer
Abstract
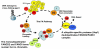
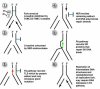
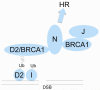
The study of rare genetic diseases can lead to insights into the cause and treatment of common diseases. An example is the rare chromosomal instability disorder, Fanconi Anemia (FA). Studies of this disease have elucidated general mechanisms of bone marrow failure, cancer pathogenesis, and resistance to chemotherapy. The principal features of FA are aplastic anemia in childhood, susceptibility to cancer or leukemia, and hypersensitivity of FA cells to DNA cross-linking agents. There are thirteen FA genes, and one of these genes is identical to the well known breast cancer susceptibility gene, BRCA2. The corresponding FA proteins cooperate in the recognition and repair of damaged DNA. Inactivation of FA genes occurs not only in FA patients but also in a variety of cancers in the general population. These findings have broad implications for predicting the sensitivity and resistance of tumors to conventional anti-cancer agents, to inhibitors of poly-ADP ribose polymerase 1, an enzyme involved in DNA repair, and to other inhibitors of DNA repair.
Figures

Similar articles
-
Fanconi anemia and breast cancer susceptibility.Nat Genet. 2007 Feb;39(2):142-3. doi: 10.1038/ng0207-142. Nat Genet. 2007. PMID: 17262024 No abstract available.
-
Connecting Fanconi's anaemia to breast cancer predisposition.Lancet. 2002 Nov 2;360(9343):1344-5. doi: 10.1016/S0140-6736(02)11414-0. Lancet. 2002. PMID: 12423977 No abstract available.
-
A growing network of cancer-susceptibility genes.N Engl J Med. 2003 May 8;348(19):1917-9. doi: 10.1056/NEJMcibr023150. N Engl J Med. 2003. PMID: 12736286 Review. No abstract available.
-
Alpha-fetoprotein and Fanconi Anemia: Relevance to DNA Repair and Breast Cancer Susceptibility.Fetal Pediatr Pathol. 2017 Feb;36(1):49-61. doi: 10.1080/15513815.2016.1225873. Epub 2016 Oct 3. Fetal Pediatr Pathol. 2017. PMID: 27690720 Review.
-
Fanconi anemia is associated with a defect in the BRCA2 partner PALB2.Nat Genet. 2007 Feb;39(2):159-61. doi: 10.1038/ng1942. Epub 2006 Dec 31. Nat Genet. 2007. PMID: 17200672
Cited by
-
Differential methylation of circulating free DNA assessed through cfMeDiP as a new tool for breast cancer diagnosis and detection of BRCA1/2 mutation.J Transl Med. 2024 Oct 15;22(1):938. doi: 10.1186/s12967-024-05734-2. J Transl Med. 2024. PMID: 39407254 Free PMC article.
-
Deciphering the role of post-translational modifications in fanconi anemia proteins and their influence on tumorigenesis.Cancer Gene Ther. 2024 Aug;31(8):1113-1123. doi: 10.1038/s41417-024-00797-1. Epub 2024 Jun 15. Cancer Gene Ther. 2024. PMID: 38879655 Review.
-
Liver abnormalities are frequent and persistent in patients with Fanconi anemia.Blood Adv. 2024 Mar 26;8(6):1427-1438. doi: 10.1182/bloodadvances.2023012215. Blood Adv. 2024. PMID: 38231120 Free PMC article.
-
Identification of Multiple Bowen's Disease Skin Lesions by Careful Physical Examination in a Patient With Fanconi Anemia.Cureus. 2023 Dec 6;15(12):e50016. doi: 10.7759/cureus.50016. eCollection 2023 Dec. Cureus. 2023. PMID: 38186461 Free PMC article.
-
Establishing a novel Fanconi anemia signaling pathway-associated prognostic model and tumor clustering for pediatric acute myeloid leukemia patients.Open Med (Wars). 2023 Nov 9;18(1):20230847. doi: 10.1515/med-2023-0847. eCollection 2023. Open Med (Wars). 2023. PMID: 38025539 Free PMC article.
References
-
- Alter BP. Diagnosis, genetics, and management of inherited bone marrow failure syndromes. Hematology Am Soc Hematol Educ Program. 2007;2007:29–39. - PubMed
-
- Bagby GC, Alter BP. Fanconi anemia. Semin Hematol. 2006;43(3):147–56. - PubMed
-
- Wang W. Emergence of a DNA-damage response network consisting of Fanconi anaemia and BRCA proteins. Nat Rev Genet. 2007;8(10):735–48. - PubMed
-
- Auerbach AD. Fanconi anemia diagnosis and the diepoxybutane (DEB) test [editorial] Experimental Hematology. 1993;21(6):731–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA092584-09/CA/NCI NIH HHS/United States
- R37 HL052725/HL/NHLBI NIH HHS/United States
- R01 HL052725/HL/NHLBI NIH HHS/United States
- U19A1067751/PHS HHS/United States
- U19 AI067751/AI/NIAID NIH HHS/United States
- P01 CA092584/CA/NCI NIH HHS/United States
- R01HL52725/HL/NHLBI NIH HHS/United States
- P01CA092584/CA/NCI NIH HHS/United States
- R01DK43889/DK/NIDDK NIH HHS/United States
- R01 DK043889-18/DK/NIDDK NIH HHS/United States
- U19 AI067751-05/AI/NIAID NIH HHS/United States
- R01 DK043889/DK/NIDDK NIH HHS/United States
- R37 HL052725-17/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

