Extranuclear functions of ER impact invasive migration and metastasis by breast cancer cells
- PMID: 20460518
- PMCID: PMC2889925
- DOI: 10.1158/0008-5472.CAN-09-3834
Extranuclear functions of ER impact invasive migration and metastasis by breast cancer cells
Abstract
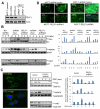
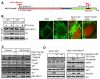
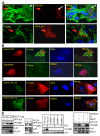
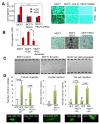
The molecular basis of breast cancer progression to metastasis and the role of estrogen receptor (ER) signaling in this process remain poorly understood. Emerging evidence suggests that ER participates in extranuclear signaling in addition to genomic functions. Recent studies identified proline-, glutamic acid-, and leucine-rich protein-1 (PELP1) as one of the components of ER signalosome in the cytoplasm. PELP1 expression is deregulated in metastatic breast tumors. We examined the mechanism and significance of ER-PELP1-mediated extranuclear signals in the cytoskeletal remodeling and metastasis. Using estrogen dendrimer conjugate (EDC) that uniquely activate ER extranuclear signaling and by using model cells that stably express PELP1 short hairpin RNA (shRNA), we show that PELP1 is required for optimal activation of ER extranuclear actions. Using a yeast two-hybrid screen, we identified integrin-linked kinase 1 (ILK1) as a novel PELP1-binding protein. Activation of extranuclear signaling by EDC uniquely enhanced E2-mediated ruffles and filopodia-like structures. Using dominant-negative and dominant-active reagents, we found that estrogen-mediated extranuclear signaling promotes cytoskeleton reorganization through the ER-Src-PELP1-phosphoinositide 3-kinase-ILK1 pathway. Using in vitro Boyden chamber assays and in vivo xenograft assays, we found that ER extranuclear actions contribute to cell migration. Collectively, our results suggest that ER extranuclear actions play a role in cell motility/metastasis, establishing for the first time that endogenous PELP1 serves as a critical component of ER extranuclear actions leading to cell motility/invasion and that the ER-Src-PELP1-ILK1 pathway represents a novel therapeutic target for preventing the emergence of ER-positive metastasis.
(c)2010 AACR.
Figures

Similar articles
-
Functional implications of altered subcellular localization of PELP1 in breast cancer cells.Cancer Res. 2005 Sep 1;65(17):7724-32. doi: 10.1158/0008-5472.CAN-05-0614. Cancer Res. 2005. PMID: 16140940 Free PMC article.
-
Modulation of in situ estrogen synthesis by proline-, glutamic acid-, and leucine-rich protein-1: potential estrogen receptor autocrine signaling loop in breast cancer cells.Mol Endocrinol. 2008 Mar;22(3):649-64. doi: 10.1210/me.2007-0350. Epub 2007 Dec 13. Mol Endocrinol. 2008. PMID: 18079323 Free PMC article.
-
Significance of PELP1 in ER-negative breast cancer metastasis.Mol Cancer Res. 2012 Jan;10(1):25-33. doi: 10.1158/1541-7786.MCR-11-0456. Epub 2011 Nov 15. Mol Cancer Res. 2012. PMID: 22086908 Free PMC article.
-
Emerging significance of ER-coregulator PELP1/MNAR in cancer.Histol Histopathol. 2007 Jan;22(1):91-6. doi: 10.14670/HH-22.91. Histol Histopathol. 2007. PMID: 17128415 Free PMC article. Review.
-
Comprehensive analysis of recent biochemical and biologic findings regarding a newly discovered protein-PELP1/MNAR.Clin Exp Metastasis. 2006;23(1):1-7. doi: 10.1007/s10585-006-9019-9. Epub 2006 Jul 7. Clin Exp Metastasis. 2006. PMID: 16826428 Review.
Cited by
-
Tumour-intrinsic PDL1 signals regulate the Chk2 DNA damage response in cancer cells and mediate resistance to Chk1 inhibitors.Mol Cancer. 2024 Oct 30;23(1):242. doi: 10.1186/s12943-024-02147-z. Mol Cancer. 2024. PMID: 39478560 Free PMC article.
-
Short-Term Exposure to Foodborne Xenoestrogens Affects Breast Cancer Cell Morphology and Motility Relevant for Metastatic Behavior In Vitro.Chem Res Toxicol. 2024 Oct 21;37(10):1634-1650. doi: 10.1021/acs.chemrestox.4c00061. Epub 2024 Sep 11. Chem Res Toxicol. 2024. PMID: 39262136 Free PMC article.
-
Association of Cytidine Deaminase Polymorphism with Capecitabine Effectiveness in Breast Cancer Patients.Asian Pac J Cancer Prev. 2023 Dec 1;24(12):4219-4225. doi: 10.31557/APJCP.2023.24.12.4219. Asian Pac J Cancer Prev. 2023. PMID: 38156857 Free PMC article.
-
Targeting nuclear hormone receptors for the prevention of breast cancer.Front Med (Lausanne). 2023 Jul 31;10:1200947. doi: 10.3389/fmed.2023.1200947. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37583424 Free PMC article. Review.
-
Role of Estrogen Receptor-Positive/Negative Ratios in Regulating Breast Cancer.Evid Based Complement Alternat Med. 2022 Sep 8;2022:7833389. doi: 10.1155/2022/7833389. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36118080 Free PMC article.
References
-
- Ariazi EA, Ariazi JL, Cordera F, Jordan VC. Estrogen receptors as therapeutic targets in breast cancer. Curr Top Med Chem. 2006;6:195–216. - PubMed
-
- Harrell JC, Dye WW, Allred DC, Jedlicka P, Spoelstra NS, Sartorius CA, et al. Estrogen receptor positive breast cancer metastasis: altered hormonal sensitivity and tumor aggressiveness in lymphatic vessels and lymph nodes. Cancer Res. 2006;66:9308–15. - PubMed
-
- Utsumi T, Kobayashi N, Hanada H. Recent perspectives of endocrine therapy for breast cancer. Breast Cancer. 2007;14:194–9. - PubMed
-
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. - PubMed
-
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous


