Molecular basis of infrared detection by snakes
- PMID: 20228791
- PMCID: PMC2855400
- DOI: 10.1038/nature08943
Molecular basis of infrared detection by snakes
Abstract
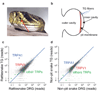
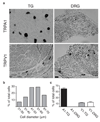
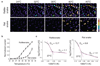
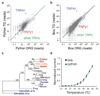
Snakes possess a unique sensory system for detecting infrared radiation, enabling them to generate a 'thermal image' of predators or prey. Infrared signals are initially received by the pit organ, a highly specialized facial structure that is innervated by nerve fibres of the somatosensory system. How this organ detects and transduces infrared signals into nerve impulses is not known. Here we use an unbiased transcriptional profiling approach to identify TRPA1 channels as infrared receptors on sensory nerve fibres that innervate the pit organ. TRPA1 orthologues from pit-bearing snakes (vipers, pythons and boas) are the most heat-sensitive vertebrate ion channels thus far identified, consistent with their role as primary transducers of infrared stimuli. Thus, snakes detect infrared signals through a mechanism involving radiant heating of the pit organ, rather than photochemical transduction. These findings illustrate the broad evolutionary tuning of transient receptor potential (TRP) channels as thermosensors in the vertebrate nervous system.
Figures
Similar articles
-
Infrared snake eyes: TRPA1 and the thermal sensitivity of the snake pit organ.Sci Signal. 2010 Jun 22;3(127):pe22. doi: 10.1126/scisignal.3127pe22. Sci Signal. 2010. PMID: 20571127
-
Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats.Nature. 2011 Aug 3;476(7358):88-91. doi: 10.1038/nature10245. Nature. 2011. PMID: 21814281 Free PMC article.
-
Molecular convergence of infrared vision in snakes.Mol Biol Evol. 2011 Jan;28(1):45-8. doi: 10.1093/molbev/msq267. Epub 2010 Oct 11. Mol Biol Evol. 2011. PMID: 20937734 Free PMC article.
-
The G protein-coupled receptor-transient receptor potential channel axis: molecular insights for targeting disorders of sensation and inflammation.Pharmacol Rev. 2015;67(1):36-73. doi: 10.1124/pr.114.009555. Pharmacol Rev. 2015. PMID: 25361914 Review.
-
TRPA1 antagonists as potential analgesic drugs.Pharmacol Ther. 2012 Feb;133(2):189-204. doi: 10.1016/j.pharmthera.2011.10.008. Epub 2011 Nov 15. Pharmacol Ther. 2012. PMID: 22119554 Review.
Cited by
-
Role of TRPA1 in Painful Cold Hypersensitivity.Adv Exp Med Biol. 2024;1461:245-252. doi: 10.1007/978-981-97-4584-5_17. Adv Exp Med Biol. 2024. PMID: 39289286 Review.
-
Thermal infrared directs host-seeking behaviour in Aedes aegypti mosquitoes.Nature. 2024 Sep;633(8030):615-623. doi: 10.1038/s41586-024-07848-5. Epub 2024 Aug 21. Nature. 2024. PMID: 39169183 Free PMC article.
-
Comparative genomics sheds new light on the convergent evolution of infrared vision in snakes.Proc Biol Sci. 2024 Aug;291(2027):20240818. doi: 10.1098/rspb.2024.0818. Epub 2024 Jul 24. Proc Biol Sci. 2024. PMID: 39043244
-
Evolution of Sensory Receptors.Annu Rev Cell Dev Biol. 2024 Oct;40(1):353-379. doi: 10.1146/annurev-cellbio-120123-112853. Epub 2024 Sep 21. Annu Rev Cell Dev Biol. 2024. PMID: 38985841 Free PMC article. Review.
-
Mammalian lures monitored with time-lapse cameras increase detection of pythons and other snakes.PeerJ. 2024 Jun 24;12:e17577. doi: 10.7717/peerj.17577. eCollection 2024. PeerJ. 2024. PMID: 38938602 Free PMC article.
References
-
- Bullock TH, Cowles RB. Physiology of an Infrared Receptor: The Facial Pit of Pit Vipers. Science. 1952;115:541–543. - PubMed
-
- Campbell AL, Naik RR, Sowards L, Stone MO. Biological infrared imaging and sensing. Micron. 2002;33:211–225. - PubMed
-
- Ebert J. Dr. rer. nat. thesis. Rheinische Friedrich Wilhelms University of Bonn; 2007. Infrared sense in snakes - behavioural and anatomical examinations (Crotalus atrox, Python regius, Corallus hortulanus)
-
- Barrett R, Maderson PFA, Meszler RM. In: Biology of Reptilia. Parsons TS, editor. Ch. 4. Academic Press; 1970. pp. 277–300.
-
- Ebert J, Schmitz A. In: Herpetologia Bonnensis II. Vences M, Kohler J, Ziegler T, Bohme W, editors. 2006. pp. 215–217.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- F32 GM080853/GM/NIGMS NIH HHS/United States
- R37 NS047723-18/NS/NINDS NIH HHS/United States
- P40 RR018300/RR/NCRR NIH HHS/United States
- NS047723/NS/NINDS NIH HHS/United States
- R01 NS055299-04S1/NS/NINDS NIH HHS/United States
- R01 NS047723/NS/NINDS NIH HHS/United States
- P40 RR018300-06/RR/NCRR NIH HHS/United States
- R01 NS055299-04/NS/NINDS NIH HHS/United States
- R01 NS055299/NS/NINDS NIH HHS/United States
- Howard Hughes Medical Institute/United States
- GM080853/GM/NIGMS NIH HHS/United States
- R37 NS047723/NS/NINDS NIH HHS/United States
- R37 NS047723-19/NS/NINDS NIH HHS/United States
- NS055299/NS/NINDS NIH HHS/United States
- P01 AG010770/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases


