Indolyne and aryne distortions and nucleophilic regioselectivites
- PMID: 20058924
- PMCID: PMC2819077
- DOI: 10.1021/ja9098643
Indolyne and aryne distortions and nucleophilic regioselectivites
Abstract
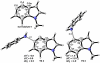
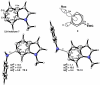
Density functional theory computations reproduce the surprisingly high regioselectivities in nucleophilic additions and cycloadditions to 4,5-indolynes and the low regioselectivities in the reactions of 5,6-indolynes. Transition-state distortion energies control the regioselectivities, activating the 5 and 6 positions over the 4 and 7 positions, leading to high preferences for 5- and 6-substituted products from 4,5- and 6,7-indolynes, respectively. Orbital and electrostatic interactions have only minor effects, producing low regioselectivities in the reactions of 5,6-indolynes. The distortion model predicts high regioselectivities with 6,7-indolynes; these have been verified experimentally. The regioselectivities found with other arynes are explained on the basis of distortion energies that are reflected in reactant geometries.
Figures
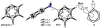
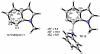
Similar articles
-
Indolyne experimental and computational studies: synthetic applications and origins of selectivities of nucleophilic additions.J Am Chem Soc. 2010 Dec 22;132(50):17933-44. doi: 10.1021/ja1086485. Epub 2010 Nov 29. J Am Chem Soc. 2010. PMID: 21114321 Free PMC article.
-
Computational predictions of substituted benzyne and indolyne regioselectivities.Tetrahedron Lett. 2015 Jun 3;56(23):3511-3514. doi: 10.1016/j.tetlet.2015.01.022. Tetrahedron Lett. 2015. PMID: 26034336 Free PMC article.
-
Overturning indolyne regioselectivities and synthesis of indolactam V.J Am Chem Soc. 2011 Mar 23;133(11):3832-5. doi: 10.1021/ja200437g. Epub 2011 Feb 25. J Am Chem Soc. 2011. PMID: 21351773 Free PMC article.
-
Pyridynes and indolynes as building blocks for functionalized heterocycles and natural products.Chem Commun (Camb). 2015 Jan 4;51(1):34-45. doi: 10.1039/c4cc06445c. Chem Commun (Camb). 2015. PMID: 25226878 Review.
-
Aromatic interactions in model systems.Curr Opin Chem Biol. 2002 Dec;6(6):736-41. doi: 10.1016/s1367-5931(02)00359-9. Curr Opin Chem Biol. 2002. PMID: 12470725 Review.
Cited by
-
Ligand-Induced Regioselectivity in Metal-Catalyzed Aryne Reactions Using Borylaryl Triflates as Aryne Precursors.Organometallics. 2023 May 22;42(10):859-864. doi: 10.1021/acs.organomet.3c00103. Epub 2023 May 10. Organometallics. 2023. PMID: 39483989 Free PMC article.
-
Derivatization of 2,1,3-Benzothiadiazole via Regioselective C-H Functionalization and Aryne Reactivity.J Org Chem. 2024 May 3;89(9):6138-6148. doi: 10.1021/acs.joc.4c00122. Epub 2024 Apr 22. J Org Chem. 2024. PMID: 38648018 Free PMC article.
-
1,3-Butadiynamides the Ethynylogous Ynamides: Synthesis, Properties and Applications in Heterocyclic Chemistry.Molecules. 2023 Jun 5;28(11):4564. doi: 10.3390/molecules28114564. Molecules. 2023. PMID: 37299038 Free PMC article. Review.
-
Asymmetric reactions involving aryne intermediates.Nat Rev Chem. 2023 Jul;7(7):496-510. doi: 10.1038/s41570-023-00485-y. Epub 2023 Mar 31. Nat Rev Chem. 2023. PMID: 37117814 Review.
-
Strain-promoted reactions of 1,2,3-cyclohexatriene and its derivatives.Nature. 2023 Jun;618(7966):748-754. doi: 10.1038/s41586-023-06075-8. Epub 2023 Apr 19. Nature. 2023. PMID: 37075803 Free PMC article.
References
-
- Bandini M, Eichholzer A. Angew. Chem. Int. Ed. 2009;48:9608–9644. - PubMed
-
- Tian X, Huters AD, Douglas CJ, Garg NK. Org. Lett. 2009;11:2349–2351. - PubMed
-
- Bronner SM, Bahnck KB, Garg NK. Org. Lett. 2009;11:1007–1010. - PubMed
-
- Buszek KR, Brown N, Luo D. Org. Lett. 2009;11:201–204. - PMC - PubMed
- Brown N, Luo D, Decapo JA, Buszek KR. Tetrahedron Lett. 2009;50:7113–7115. - PMC - PubMed
- Buszek KR, Luo D, Kondrashov M, Brown N, VanderVelde D. Org. Lett. 2007;9:4135–4137. - PubMed
- Brown N, Luo D, VanderVelde D, Yang S, Brassfield A, Buszek KR. Tetrahedron Lett. 2009;50:63–65. - PMC - PubMed
-
-
Also known as deformation energy:Nagase S, Morokuma K. J. Am. Chem. Soc. 1978;100:1666–1672.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources


