A key role for orexin in panic anxiety
- PMID: 20037593
- PMCID: PMC2832844
- DOI: 10.1038/nm.2075
A key role for orexin in panic anxiety
Abstract
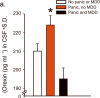
Panic disorder is a severe anxiety disorder with recurrent, debilitating panic attacks. In individuals with panic disorder there is evidence of decreased central gamma-aminobutyric acid (GABA) activity as well as marked increases in autonomic and respiratory responses after intravenous infusions of hypertonic sodium lactate. In a rat model of panic disorder, chronic inhibition of GABA synthesis in the dorsomedial-perifornical hypothalamus of rats produces anxiety-like states and a similar vulnerability to sodium lactate-induced cardioexcitatory responses. The dorsomedial-perifornical hypothalamus is enriched in neurons containing orexin (ORX, also known as hypocretin), which have a crucial role in arousal, vigilance and central autonomic mobilization, all of which are key components of panic. Here we show that activation of ORX-synthesizing neurons is necessary for developing a panic-prone state in the rat panic model, and either silencing of the hypothalamic gene encoding ORX (Hcrt) with RNAi or systemic ORX-1 receptor antagonists blocks the panic responses. Moreover, we show that human subjects with panic anxiety have elevated levels of ORX in the cerebrospinal fluid compared to subjects without panic anxiety. Taken together, our results suggest that the ORX system may be involved in the pathophysiology of panic anxiety and that ORX antagonists constitute a potential new treatment strategy for panic disorder.
Figures


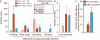
Similar articles
-
Activation of the orexin 1 receptor is a critical component of CO2-mediated anxiety and hypertension but not bradycardia.Neuropsychopharmacology. 2012 Jul;37(8):1911-22. doi: 10.1038/npp.2012.38. Epub 2012 Mar 28. Neuropsychopharmacology. 2012. PMID: 22453138 Free PMC article.
-
Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network.Physiol Behav. 2012 Dec 5;107(5):733-42. doi: 10.1016/j.physbeh.2012.04.016. Epub 2012 Apr 24. Physiol Behav. 2012. PMID: 22554617 Free PMC article.
-
Orexin, stress, and anxiety/panic states.Prog Brain Res. 2012;198:133-61. doi: 10.1016/B978-0-444-59489-1.00009-4. Prog Brain Res. 2012. PMID: 22813973 Free PMC article. Review.
-
Disinhibition of perifornical hypothalamic neurones activates noradrenergic neurones and blocks pontine carbachol-induced REM sleep-like episodes in rats.J Physiol. 2007 Jul 15;582(Pt 2):553-67. doi: 10.1113/jphysiol.2007.127613. Epub 2007 May 10. J Physiol. 2007. PMID: 17495048 Free PMC article.
-
An animal model of panic vulnerability with chronic disinhibition of the dorsomedial/perifornical hypothalamus.Physiol Behav. 2012 Dec 5;107(5):686-98. doi: 10.1016/j.physbeh.2012.03.016. Epub 2012 Mar 26. Physiol Behav. 2012. PMID: 22484112 Free PMC article. Review.
Cited by
-
Exaggerated postnatal surge of orexin neurons and the effects of elimination of excess orexin on blood pressure and exaggerated chemoreflex in spontaneously hypertensive rats.Front Physiol. 2024 Oct 9;15:1341649. doi: 10.3389/fphys.2024.1341649. eCollection 2024. Front Physiol. 2024. PMID: 39469444 Free PMC article.
-
Contribution of hypothalamic orexin (hypocretin) circuits to pathologies of motivation.Br J Pharmacol. 2024 Nov;181(22):4430-4449. doi: 10.1111/bph.17325. Epub 2024 Sep 24. Br J Pharmacol. 2024. PMID: 39317446 Review.
-
Hypocretin in the nucleus accumbens shell modulates social approach in female but not male California mice.Neuropsychopharmacology. 2024 Dec;49(13):2000-2010. doi: 10.1038/s41386-024-01937-9. Epub 2024 Aug 8. Neuropsychopharmacology. 2024. PMID: 39117901 Free PMC article.
-
Hypocretin (Orexin) Replacement Therapies.Med Drug Discov. 2020 Dec;8:100070. doi: 10.1016/j.medidd.2020.100070. Epub 2020 Oct 17. Med Drug Discov. 2020. PMID: 38738170 Free PMC article.
-
Tryptophan metabolic pathway plays a key role in the stress-induced emotional eating.Curr Res Food Sci. 2024 Apr 26;8:100754. doi: 10.1016/j.crfs.2024.100754. eCollection 2024. Curr Res Food Sci. 2024. PMID: 38736909 Free PMC article.
References
-
- Goddard AW, et al. Reductions in occipital cortex GABA levels in panic disorder detected with 1h-magnetic resonance spectroscopy. Archives of General Psychiatry. 2001;58:556–561. - PubMed
-
- Goddard AW, et al. Impaired GABA neuronal response to acute benzodiazepine administration in panic disorder. Am J Psychiatry. 2004;161:2186–2193. - PubMed
-
- Roy-Byrne PP, Craske MG, Stein MB. Panic disorder. Lancet. 2006;368:1023–1032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases


