Gla-rich protein is a novel vitamin K-dependent protein present in serum that accumulates at sites of pathological calcifications
- PMID: 19893032
- PMCID: PMC2789615
- DOI: 10.2353/ajpath.2009.090474
Gla-rich protein is a novel vitamin K-dependent protein present in serum that accumulates at sites of pathological calcifications
Abstract
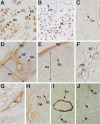
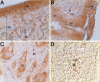
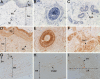
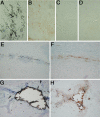
Mineralization of soft tissues is an abnormal process that occurs in any body tissue and can greatly increase morbidity and mortality. Vitamin K-dependent (VKD) proteins play a crucial role in these processes; matrix Gla protein is considered one of the most relevant physiological inhibitors of soft tissue calcification know to date. Several studies have suggested that other, still unknown, VKD proteins might also be involved in soft tissue calcification pathologies. We have recently identified in sturgeon a new VKD protein, Gla-rich protein (GRP), which contains the highest ratio between number of Gla residues and size of the mature protein so far identified. Although mainly expressed in cartilaginous tissues of sturgeon, in rat GRP is present in both cartilage and bone. We now show that GRP is a circulating protein that is also expressed and accumulated in soft tissues of rats and humans, including the skin and vascular system in which, when affected by pathological calcifications, GRP accumulates at high levels at sites of mineral deposition, indicating an association with calcification processes. The high number of Gla residues and consequent mineral binding affinity properties strongly suggest that GRP may directly influence mineral formation, thereby playing a role in processes involving connective tissue mineralization.
Figures


Similar articles
-
Gla-rich protein acts as a calcification inhibitor in the human cardiovascular system.Arterioscler Thromb Vasc Biol. 2015 Feb;35(2):399-408. doi: 10.1161/ATVBAHA.114.304823. Epub 2014 Dec 23. Arterioscler Thromb Vasc Biol. 2015. PMID: 25538207
-
Purification of matrix Gla protein from a marine teleost fish, Argyrosomus regius: calcified cartilage and not bone as the primary site of MGP accumulation in fish.J Bone Miner Res. 2003 Feb;18(2):244-59. doi: 10.1359/jbmr.2003.18.2.244. J Bone Miner Res. 2003. PMID: 12568402
-
Gla-rich protein, a new player in tissue calcification?Adv Nutr. 2012 Mar 1;3(2):174-81. doi: 10.3945/an.111.001685. Adv Nutr. 2012. PMID: 22516725 Free PMC article. Review.
-
Gla-rich protein is a potential new vitamin K target in cancer: evidences for a direct GRP-mineral interaction.Biomed Res Int. 2014;2014:340216. doi: 10.1155/2014/340216. Epub 2014 May 18. Biomed Res Int. 2014. PMID: 24949434 Free PMC article.
-
Implication of a novel vitamin K dependent protein, GRP/Ucma in the pathophysiological conditions associated with vascular and soft tissue calcification, osteoarthritis, inflammation, and carcinoma.Int J Biol Macromol. 2018 Jul 1;113:309-316. doi: 10.1016/j.ijbiomac.2018.02.150. Epub 2018 Feb 27. Int J Biol Macromol. 2018. PMID: 29499263 Review.
Cited by
-
Two Members of Vitamin-K-Dependent Proteins, Gla-Rich Protein (GRP) and Matrix Gla Protein (MGP), as Possible New Players in the Molecular Mechanism of Osteoarthritis.J Clin Med. 2024 Aug 30;13(17):5159. doi: 10.3390/jcm13175159. J Clin Med. 2024. PMID: 39274372 Free PMC article.
-
Extrahepatic Vitamin K-Dependent Gla-Proteins-Potential Cardiometabolic Biomarkers.Int J Mol Sci. 2024 Mar 20;25(6):3517. doi: 10.3390/ijms25063517. Int J Mol Sci. 2024. PMID: 38542487 Free PMC article. Review.
-
Roles of vitamin K‑dependent protein in biomineralization (Review).Int J Mol Med. 2024 Jan;53(1):6. doi: 10.3892/ijmm.2023.5330. Epub 2023 Nov 24. Int J Mol Med. 2024. PMID: 37997858 Free PMC article. Review.
-
Brain Trauma and the Secondary Cascade in Humans: Review of the Potential Role of Vitamins in Reparative Processes and Functional Outcome.Behav Sci (Basel). 2023 May 8;13(5):388. doi: 10.3390/bs13050388. Behav Sci (Basel). 2023. PMID: 37232626 Free PMC article. Review.
-
Links between Vitamin K, Ferroptosis and SARS-CoV-2 Infection.Antioxidants (Basel). 2023 Mar 16;12(3):733. doi: 10.3390/antiox12030733. Antioxidants (Basel). 2023. PMID: 36978981 Free PMC article. Review.
References
-
- Schinke T, McKee MD, Karsenty G. Extracellular matrix calcification: where is the action? Nat Genet. 1999;21:225–229. - PubMed
-
- Wallin R, Wajih N, Greenwood GT, Sane DC. Arterial calcification: a review of mechanisms, animal models, and the prospects for therapy. Med Res Rev. 2001;21:274–301. - PubMed
-
- Cranenburg EC, Schurgers LJ, Vermeer C. Vitamin K: the coagulation vitamin that became omnipotent. Thromb Haemost. 2007;98:120–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


