Fully functional bioengineered tooth replacement as an organ replacement therapy
- PMID: 19666587
- PMCID: PMC2720406
- DOI: 10.1073/pnas.0902944106
Fully functional bioengineered tooth replacement as an organ replacement therapy
Abstract
Current approaches to the development of regenerative therapies have been influenced by our understanding of embryonic development, stem cell biology, and tissue engineering technology. The ultimate goal of regenerative therapy is to develop fully functioning bioengineered organs which work in cooperation with surrounding tissues to replace organs that were lost or damaged as a result of disease, injury, or aging. Here, we report a successful fully functioning tooth replacement in an adult mouse achieved through the transplantation of bioengineered tooth germ into the alveolar bone in the lost tooth region. We propose this technology as a model for future organ replacement therapies. The bioengineered tooth, which was erupted and occluded, had the correct tooth structure, hardness of mineralized tissues for mastication, and response to noxious stimulations such as mechanical stress and pain in cooperation with other oral and maxillofacial tissues. This study represents a substantial advance and emphasizes the potential for bioengineered organ replacement in future regenerative therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


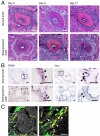
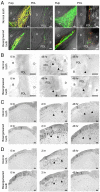
Similar articles
-
Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy.PLoS One. 2011;6(7):e21531. doi: 10.1371/journal.pone.0021531. Epub 2011 Jul 12. PLoS One. 2011. PMID: 21765896 Free PMC article.
-
Development and prospects of organ replacement regenerative therapy.Cornea. 2013 Nov;32 Suppl 1:S13-21. doi: 10.1097/ICO.0b013e3182a18e6c. Cornea. 2013. PMID: 24104927 Review.
-
Whole Tooth Regeneration as a Future Dental Treatment.Adv Exp Med Biol. 2015;881:255-69. doi: 10.1007/978-3-319-22345-2_14. Adv Exp Med Biol. 2015. PMID: 26545754 Review.
-
Practical whole-tooth restoration utilizing autologous bioengineered tooth germ transplantation in a postnatal canine model.Sci Rep. 2017 Mar 16;7:44522. doi: 10.1038/srep44522. Sci Rep. 2017. PMID: 28300208 Free PMC article.
-
The development of a bioengineered organ germ method.Nat Methods. 2007 Mar;4(3):227-30. doi: 10.1038/nmeth1012. Epub 2007 Feb 18. Nat Methods. 2007. PMID: 17322892
Cited by
-
Engineered pre-dentin with well-aligned hierarchical mineralized collagen fibril bundles promote bio-root regeneration.J Tissue Eng. 2024 Sep 27;15:20417314241280961. doi: 10.1177/20417314241280961. eCollection 2024 Jan-Dec. J Tissue Eng. 2024. PMID: 39380665 Free PMC article.
-
Bioengineering from the laboratory to clinical translation in oral and maxillofacial reconstruction.Saudi Dent J. 2024 Jul;36(7):955-962. doi: 10.1016/j.sdentj.2024.05.004. Epub 2024 May 8. Saudi Dent J. 2024. PMID: 39035556 Free PMC article. Review.
-
From Pluripotent Stem Cells to Organoids and Bioprinting: Recent Advances in Dental Epithelium and Ameloblast Models to Study Tooth Biology and Regeneration.Stem Cell Rev Rep. 2024 Jul;20(5):1184-1199. doi: 10.1007/s12015-024-10702-w. Epub 2024 Mar 18. Stem Cell Rev Rep. 2024. PMID: 38498295 Free PMC article. Review.
-
Adult Mesenchymal Stem Cells from Oral Cavity and Surrounding Areas: Types and Biomedical Applications.Pharmaceutics. 2023 Aug 9;15(8):2109. doi: 10.3390/pharmaceutics15082109. Pharmaceutics. 2023. PMID: 37631323 Free PMC article. Review.
-
Bioprinting and biomaterials for dental alveolar tissue regeneration.Front Bioeng Biotechnol. 2023 Apr 14;11:991821. doi: 10.3389/fbioe.2023.991821. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37122863 Free PMC article. Review.
References
-
- Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. - PubMed
-
- Watt FM, Hogan BL. Out of Eden: Stem cells and their niches. Science. 2000;287:1427–1430. - PubMed
-
- Langer RS, Vacanti JP. Tissue engineering: The challenges ahead. Sci Am. 1999;280:86–89. - PubMed
-
- Atala A. Tissue engineering, stem cells and cloning: Current concepts and changing trends. Expert Opin Biol Ther. 2005;5:879–892. - PubMed
-
- Korbling M, Estrov Z. Adult stem cells for tissue repair–a new therapeutic concept? N Engl J Med. 2003;349:570–582. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials


