Wnt/beta-catenin signaling: components, mechanisms, and diseases
- PMID: 19619488
- PMCID: PMC2861485
- DOI: 10.1016/j.devcel.2009.06.016
Wnt/beta-catenin signaling: components, mechanisms, and diseases
Abstract
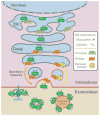
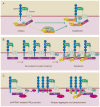
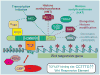
Signaling by the Wnt family of secreted glycolipoproteins via the transcriptional coactivator beta-catenin controls embryonic development and adult homeostasis. Here we review recent progress in this so-called canonical Wnt signaling pathway. We discuss Wnt ligands, agonists, and antagonists, and their interactions with Wnt receptors. We also dissect critical events that regulate beta-catenin stability, from Wnt receptors to the cytoplasmic beta-catenin destruction complex, and nuclear machinery that mediates beta-catenin-dependent transcription. Finally, we highlight some key aspects of Wnt/beta-catenin signaling in human diseases including congenital malformations, cancer, and osteoporosis, and discuss potential therapeutic implications.
Figures



Similar articles
-
Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions.Development. 2008 Jan;135(2):367-75. doi: 10.1242/dev.013540. Epub 2007 Dec 12. Development. 2008. PMID: 18077588 Free PMC article.
-
Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression.J Biol Chem. 2006 May 12;281(19):13247-13257. doi: 10.1074/jbc.M508324200. Epub 2006 Mar 16. J Biol Chem. 2006. PMID: 16543246
-
LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of beta-catenin.Proc Natl Acad Sci U S A. 2008 Jun 10;105(23):8032-7. doi: 10.1073/pnas.0803025105. Epub 2008 May 28. Proc Natl Acad Sci U S A. 2008. PMID: 18509060 Free PMC article.
-
Molecular bases of the regulation of bone remodeling by the canonical Wnt signaling pathway.Curr Top Dev Biol. 2006;73:43-84. doi: 10.1016/S0070-2153(05)73002-7. Curr Top Dev Biol. 2006. PMID: 16782455 Review.
-
Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know?Endocr Rev. 2005 Dec;26(7):898-915. doi: 10.1210/er.2003-0034. Epub 2005 Aug 26. Endocr Rev. 2005. PMID: 16126938 Review.
Cited by
-
Effect of bioactive compounds of Mucuna pruriens on proteins of Wnt/β catenin pathway in pulmonary hypertension by in silico approach.In Silico Pharmacol. 2024 Nov 19;12(2):110. doi: 10.1007/s40203-024-00263-8. eCollection 2024. In Silico Pharmacol. 2024. PMID: 39575208
-
Polygenic anti-cancer activity of Indigofera macrophylla in prostate cancer induced animal model.Toxicol Rep. 2024 Oct 22;13:101774. doi: 10.1016/j.toxrep.2024.101774. eCollection 2024 Dec. Toxicol Rep. 2024. PMID: 39554609 Free PMC article.
-
The multifaceted anticancer potential of luteolin: involvement of NF-κB, AMPK/mTOR, PI3K/Akt, MAPK, and Wnt/β-catenin pathways.Inflammopharmacology. 2024 Nov 14. doi: 10.1007/s10787-024-01596-8. Online ahead of print. Inflammopharmacology. 2024. PMID: 39543054 Review.
-
PLEKHA4 upregulation regulates KIRC cell proliferation through β‑catenin signaling.Mol Med Rep. 2025 Jan;31(1):30. doi: 10.3892/mmr.2024.13395. Epub 2024 Nov 14. Mol Med Rep. 2025. PMID: 39540374 Free PMC article.
-
The Functional Role of the Long Non-Coding RNA LINCMD1 in Leiomyoma Pathogenesis.Int J Mol Sci. 2024 Oct 27;25(21):11539. doi: 10.3390/ijms252111539. Int J Mol Sci. 2024. PMID: 39519092 Free PMC article.
References
-
- Angers S, Thorpe CJ, Biechele TL, Goldenberg SJ, Zheng N, MacCoss MJ, Moon RT. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nature cell biology. 2006;8:348–357. - PubMed
-
- Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25:7492–7504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases


