Characterization of the tumor marker muc16 (ca125) expressed by murine ovarian tumor cell lines and identification of a panel of cross-reactive monoclonal antibodies
- PMID: 19538730
- PMCID: PMC2708168
- DOI: 10.1186/1757-2215-2-8
Characterization of the tumor marker muc16 (ca125) expressed by murine ovarian tumor cell lines and identification of a panel of cross-reactive monoclonal antibodies
Abstract
Objectives: The ovarian tumor marker CA125 is expressed on human MUC16, a cell surface bound mucin that is also shed by proteolytic cleavage. Human MUC16 is overexpressed by ovarian cancer cells. MUC16 facilitates the binding of ovarian tumor cells to mesothelial cells lining the peritoneal cavity. Additionally, MUC16 also is a potent inhibitor of natural killer cell mediated anti-tumor cytotoxic responses. Extensive studies using human as well as murine ovarian tumor cell models are required to clearly define the function of MUC16 in the progression of ovarian tumors. The major objective of this study was to determine if the murine ovarian tumor cells, MOVCAR, express Muc16 and to characterize antibodies that recognize this mucin.
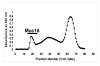
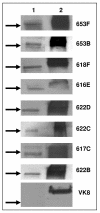
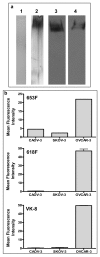
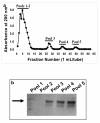
Methods: RT-PCR analysis was used for detecting the Muc16 message and size exclusion column chromatography for isolating Muc16 produced by MOVCAR cells. Soluble and cell-associated murine Muc16 were analyzed, respectively, by Western blotting and flow cytometry assays using a new panel of antibodies. The presence of N-linked oligosaccharides on murine Muc16 was determined by ConA chromatography.
Results: We demonstrate that murine Muc16 is expressed by mouse ovarian cancer cells as an ~250 kDa glycoprotein that carries both O-linked and N-linked oligosaccharides. In contrast to human MUC16, the murine ortholog is primarily released from the cells and cannot be detected on the cell surface. Since the released murine Muc16 is not detected by conventional anti-CA125 assays, we have for the first time identified a panel of anti-human MUC16 antibodies that also recognizes the murine counterpart.
Conclusion: The antibodies identified in this study can be used in future purification of murine Muc16 and exhaustive study of its properties. Furthermore, the initial identification and characterization of murine Muc16 is a vital preliminary step in the development of effective murine models of human ovarian cancer. These models will aid in the further elucidation of the role that human MUC16 plays in the etiology and progression of ovarian tumors.
Figures

Similar articles
-
Development and characterization of carboxy-terminus specific monoclonal antibodies for understanding MUC16 cleavage in human ovarian cancer.PLoS One. 2018 Apr 30;13(4):e0193907. doi: 10.1371/journal.pone.0193907. eCollection 2018. PLoS One. 2018. PMID: 29708979 Free PMC article.
-
Virus-like particle vaccine displaying an external, membrane adjacent MUC16 epitope elicits ovarian cancer-reactive antibodies.J Ovarian Res. 2024 Jan 16;17(1):19. doi: 10.1186/s13048-023-01325-9. J Ovarian Res. 2024. PMID: 38225646 Free PMC article.
-
MUC16 expression during embryogenesis, in adult tissues, and ovarian cancer in the mouse.Differentiation. 2008 Dec;76(10):1081-92. doi: 10.1111/j.1432-0436.2008.00295.x. Epub 2008 Jul 2. Differentiation. 2008. PMID: 18637025 Free PMC article.
-
Peritoneal dissemination of ovarian cancer: role of MUC16-mesothelin interaction and implications for treatment.Expert Rev Anticancer Ther. 2018 Feb;18(2):177-186. doi: 10.1080/14737140.2018.1418326. Epub 2017 Dec 21. Expert Rev Anticancer Ther. 2018. PMID: 29241375 Review.
-
[MUC16: The Novel Target for Tumor Therapy].Zhongguo Fei Ai Za Zhi. 2022 Jul 20;25(7):452-459. doi: 10.3779/j.issn.1009-3419.2022.101.31. Zhongguo Fei Ai Za Zhi. 2022. PMID: 35899441 Free PMC article. Review. Chinese.
Cited by
-
Ion mobility-tandem mass spectrometry of mucin-type O-glycans.Nat Commun. 2024 Mar 23;15(1):2611. doi: 10.1038/s41467-024-46825-4. Nat Commun. 2024. PMID: 38521783 Free PMC article.
-
Chimeric antibody targeting unique epitope on onco-mucin16 reduces tumor burden in pancreatic and lung malignancies.NPJ Precis Oncol. 2023 Aug 11;7(1):74. doi: 10.1038/s41698-023-00423-7. NPJ Precis Oncol. 2023. PMID: 37567918 Free PMC article.
-
Fabrication of mesoporous silica nanoparticles for targeted delivery of sunitinib to ovarian cancer cells.Bioimpacts. 2023;13(3):255-267. doi: 10.34172/bi.2023.25298. Epub 2023 Jan 23. Bioimpacts. 2023. PMID: 37431477 Free PMC article.
-
Lysosomal cathepsin D mediates endogenous mucin glycodomain catabolism in mammals.Proc Natl Acad Sci U S A. 2022 Sep 27;119(39):e2117105119. doi: 10.1073/pnas.2117105119. Epub 2022 Sep 19. Proc Natl Acad Sci U S A. 2022. PMID: 36122205 Free PMC article.
-
Plasmonic Nanoparticle-Based Digital Cytometry to Quantify MUC16 Binding on the Surface of Leukocytes in Ovarian Cancer.ACS Sens. 2020 Sep 25;5(9):2772-2782. doi: 10.1021/acssensors.0c00567. Epub 2020 Sep 10. ACS Sens. 2020. PMID: 32847358 Free PMC article.
References
-
- Bast RC, Jr, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med . 1983;309:883–887. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous


