Effects of pH and iminosugar pharmacological chaperones on lysosomal glycosidase structure and stability
- PMID: 19374450
- PMCID: PMC2699628
- DOI: 10.1021/bi9002265
Effects of pH and iminosugar pharmacological chaperones on lysosomal glycosidase structure and stability
Abstract
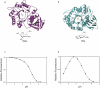
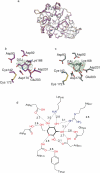
Human lysosomal enzymes acid-beta-glucosidase (GCase) and acid-alpha-galactosidase (alpha-Gal A) hydrolyze the sphingolipids glucosyl- and globotriaosylceramide, respectively, and mutations in these enzymes lead to the lipid metabolism disorders Gaucher and Fabry disease, respectively. We have investigated the structure and stability of GCase and alpha-Gal A in a neutral-pH environment reflective of the endoplasmic reticulum and an acidic-pH environment reflective of the lysosome. These details are important for the development of pharmacological chaperone therapy for Gaucher and Fabry disease, in which small molecules bind mutant enzymes in the ER to enable the mutant enzyme to meet quality control requirements for lysosomal trafficking. We report crystal structures of apo GCase at pH 4.5, at pH 5.5, and in complex with the pharmacological chaperone isofagomine (IFG) at pH 7.5. We also present thermostability analysis of GCase at pH 7.4 and 5.2 using differential scanning calorimetry. We compare our results with analogous experiments using alpha-Gal A and the chaperone 1-deoxygalactonijirimycin (DGJ), including the first structure of alpha-Gal A with DGJ. Both GCase and alpha-Gal A are more stable at lysosomal pH with and without their respective iminosugars bound, and notably, the stability of the GCase-IFG complex is pH sensitive. We show that the conformations of the active site loops in GCase are sensitive to ligand binding but not pH, whereas analogous galactose- or DGJ-dependent conformational changes in alpha-Gal A are not seen. Thermodynamic parameters obtained from alpha-Gal A unfolding indicate two-state, van't Hoff unfolding in the absence of the iminosugar at neutral and lysosomal pH, and non-two-state unfolding in the presence of DGJ. Taken together, these results provide insight into how GCase and alpha-Gal A are thermodynamically stabilized by iminosugars and suggest strategies for the development of new pharmacological chaperones for lysosomal storage disorders.
Figures
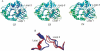
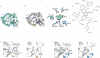
Similar articles
-
Pharmacological chaperone corrects lysosomal storage in Fabry disease caused by trafficking-incompetent variants.Am J Physiol Cell Physiol. 2006 Apr;290(4):C1076-82. doi: 10.1152/ajpcell.00426.2005. Am J Physiol Cell Physiol. 2006. PMID: 16531566
-
Structure of acid beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease.Nat Chem Biol. 2007 Feb;3(2):101-7. doi: 10.1038/nchembio850. Epub 2006 Dec 24. Nat Chem Biol. 2007. PMID: 17187079
-
The pharmacological chaperone 1-deoxygalactonojirimycin increases alpha-galactosidase A levels in Fabry patient cell lines.J Inherit Metab Dis. 2009 Jun;32(3):424-40. doi: 10.1007/s10545-009-1077-0. Epub 2009 Apr 18. J Inherit Metab Dis. 2009. PMID: 19387866
-
Computational and modeling approaches to understand the impact of the Fabry's disease causing mutation (D92Y) on the interaction with pharmacological chaperone 1-deoxygalactonojirimycin (DGJ).Adv Protein Chem Struct Biol. 2019;114:341-407. doi: 10.1016/bs.apcsb.2018.10.009. Epub 2018 Dec 18. Adv Protein Chem Struct Biol. 2019. PMID: 30635085 Review.
-
Chaperone therapy update: Fabry disease, GM1-gangliosidosis and Gaucher disease.Brain Dev. 2013 Jun;35(6):515-23. doi: 10.1016/j.braindev.2012.12.002. Epub 2013 Jan 3. Brain Dev. 2013. PMID: 23290321 Review.
Cited by
-
Mechanistic Insights into Dibasic Iminosugars as pH-Selective Pharmacological Chaperones to Stabilize Human α-Galactosidase.JACS Au. 2024 Feb 23;4(3):908-918. doi: 10.1021/jacsau.3c00684. eCollection 2024 Mar 25. JACS Au. 2024. PMID: 38559739 Free PMC article.
-
Genetic variations in GBA1 and LRRK2 genes: Biochemical and clinical consequences in Parkinson disease.Front Neurol. 2022 Aug 12;13:971252. doi: 10.3389/fneur.2022.971252. eCollection 2022. Front Neurol. 2022. PMID: 36034282 Free PMC article. Review.
-
sp2-Iminosugars targeting human lysosomal β-hexosaminidase as pharmacological chaperone candidates for late-onset Tay-Sachs disease.J Enzyme Inhib Med Chem. 2022 Dec;37(1):1364-1374. doi: 10.1080/14756366.2022.2073444. J Enzyme Inhib Med Chem. 2022. PMID: 35575117 Free PMC article.
-
Fabry disease - a multisystemic disease with gastrointestinal manifestations.Gut Microbes. 2022 Jan-Dec;14(1):2027852. doi: 10.1080/19490976.2022.2027852. Gut Microbes. 2022. PMID: 35090382 Free PMC article. Review.
-
Design, Synthesis and Structural Analysis of Glucocerebrosidase Imaging Agents.Chemistry. 2021 Nov 25;27(66):16377-16388. doi: 10.1002/chem.202102359. Epub 2021 Oct 29. Chemistry. 2021. PMID: 34570911 Free PMC article.
References
-
- Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell. Biol. 2004;5:554–565. - PubMed
-
- Grace ME, Newman KM, Scheinker V, Berg-Fussman A, Grabowski GA. Analysis of human acid beta-glucosidase by site-directed mutagenesis and heterologous expression. J. Biol. Chem. 1994;269:2283–2291. - PubMed
-
- Liou B, Kazimierczuk A, Zhang M, Scott CR, Hegde RS, Grabowski GA. Analyses of variant acid beta -glucosidases: effects of gaucher disease mutations. J. Biol. Chem. 2006;281:4242–4253. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


