Toxin B is essential for virulence of Clostridium difficile
- PMID: 19252482
- PMCID: PMC2679968
- DOI: 10.1038/nature07822
Toxin B is essential for virulence of Clostridium difficile
Abstract
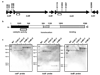
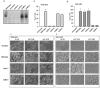
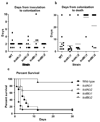
Clostridium difficile is the leading cause of infectious diarrhoea in hospitals worldwide, because of its virulence, spore-forming ability and persistence. C. difficile-associated diseases are induced by antibiotic treatment or disruption of the normal gastrointestinal flora. Recently, morbidity and mortality resulting from C. difficile-associated diseases have increased significantly due to changes in the virulence of the causative strains and antibiotic usage patterns. Since 2002, epidemic toxinotype III NAP1/027 strains, which produce high levels of the major virulence factors, toxin A and toxin B, have emerged. These toxins have 63% amino acid sequence similarity and are members of the large clostridial glucosylating toxin family, which are monoglucosyltransferases that are pro-inflammatory, cytotoxic and enterotoxic in the human colon. Inside host cells, both toxins catalyse the transfer of glucose onto the Rho family of GTPases, leading to cell death. However, the role of these toxins in the context of a C. difficile infection is unknown. Here we describe the construction of isogenic tcdA and tcdB (encoding toxin A and B, respectively) mutants of a virulent C. difficile strain and their use in the hamster disease model to show that toxin B is a key virulence determinant. Previous studies showed that purified toxin A alone can induce most of the pathology observed after infection of hamsters with C. difficile and that toxin B is not toxic in animals unless it is co-administered with toxin A, suggesting that the toxins act synergistically. Our work provides evidence that toxin B, not toxin A, is essential for virulence. Furthermore, it is clear that the importance of these toxins in the context of infection cannot be predicted exclusively from studies using purified toxins, reinforcing the importance of using the natural infection process to dissect the role of toxins in disease.
Figures
Similar articles
-
The role of toxin A and toxin B in Clostridium difficile infection.Nature. 2010 Oct 7;467(7316):711-3. doi: 10.1038/nature09397. Epub 2010 Sep 15. Nature. 2010. PMID: 20844489
-
Defining the Roles of TcdA and TcdB in Localized Gastrointestinal Disease, Systemic Organ Damage, and the Host Response during Clostridium difficile Infections.mBio. 2015 Jun 2;6(3):e00551. doi: 10.1128/mBio.00551-15. mBio. 2015. PMID: 26037121 Free PMC article.
-
Epidemic ribotypes of Clostridium (now Clostridioides) difficile are likely to be more virulent than non-epidemic ribotypes in animal models.BMC Microbiol. 2020 Feb 5;20(1):27. doi: 10.1186/s12866-020-1710-5. BMC Microbiol. 2020. PMID: 32024477 Free PMC article.
-
The role of flagella in Clostridium difficile pathogenicity.Trends Microbiol. 2015 May;23(5):275-82. doi: 10.1016/j.tim.2015.01.004. Epub 2015 Feb 4. Trends Microbiol. 2015. PMID: 25659185 Review.
-
Cyclic diguanylate differentially regulates the expression of virulence factors and pathogenesis-related phenotypes in Clostridioides difficile.Microbiol Res. 2024 Sep;286:127811. doi: 10.1016/j.micres.2024.127811. Epub 2024 Jun 20. Microbiol Res. 2024. PMID: 38909416 Review.
Cited by
-
De novo design of mini-protein binders broadly neutralizing Clostridioides difficile toxin B variants.Nat Commun. 2024 Oct 2;15(1):8521. doi: 10.1038/s41467-024-52582-1. Nat Commun. 2024. PMID: 39358329 Free PMC article.
-
Toxin-linked mobile genetic elements in major enteric bacterial pathogens.Gut Microbiome (Camb). 2023 Mar 17;4:e5. doi: 10.1017/gmb.2023.2. eCollection 2023. Gut Microbiome (Camb). 2023. PMID: 39295911 Free PMC article. Review.
-
Genomic characterization and virulence gene profiling of Erysipelothrix rhusiopathiae isolated from widespread muskox mortalities in the Canadian Arctic Archipelago.BMC Genomics. 2024 Jul 14;25(1):691. doi: 10.1186/s12864-024-10592-9. BMC Genomics. 2024. PMID: 39004696 Free PMC article.
-
Spores of Clostridioides difficile are toxin delivery vehicles.Commun Biol. 2024 Jul 10;7(1):839. doi: 10.1038/s42003-024-06521-x. Commun Biol. 2024. PMID: 38987278 Free PMC article.
-
A sequence invariable region in TcdB2 is required for toxin escape from Clostridioides difficile.J Bacteriol. 2024 Jul 25;206(7):e0009624. doi: 10.1128/jb.00096-24. Epub 2024 Jun 18. J Bacteriol. 2024. PMID: 38888328
References
-
- McDonald LC, et al. An epidemic, toxin gene–variant strain of Clostridium difficile. N. Engl. J. Med. 2005;353:2433–2441. - PubMed
-
- Warny M, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. - PubMed
-
- Bartlett JG, et al. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978;75:778–782. - PubMed
-
- Bartlett JG. Antibiotic-associated diarrhea. N. Engl. J. Med. 2002;346:334–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


