An oxyferrous heme/protein-based radical intermediate is catalytically competent in the catalase reaction of Mycobacterium tuberculosis catalase-peroxidase (KatG)
- PMID: 19139099
- PMCID: PMC2652337
- DOI: 10.1074/jbc.M808106200
An oxyferrous heme/protein-based radical intermediate is catalytically competent in the catalase reaction of Mycobacterium tuberculosis catalase-peroxidase (KatG)
Abstract
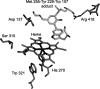
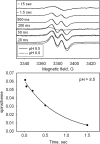
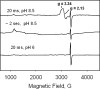
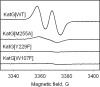
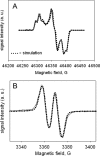
A mechanism accounting for the robust catalase activity in catalase-peroxidases (KatG) presents a new challenge in heme protein enzymology. In Mycobacterium tuberculosis, KatG is the sole catalase and is also responsible for peroxidative activation of isoniazid, an anti-tuberculosis pro-drug. Here, optical stopped-flow spectrophotometry, rapid freeze-quench EPR spectroscopy both at the X-band and at the D-band, and mutagenesis are used to identify catalase reaction intermediates in M. tuberculosis KatG. In the presence of millimolar H2O2 at neutral pH, oxyferrous heme is formed within milliseconds from ferric (resting) KatG, whereas at pH 8.5, low spin ferric heme is formed. Using rapid freeze-quench EPR at X-band under both of these conditions, a narrow doublet radical signal with an 11 G principal hyperfine splitting was detected within the first milliseconds of turnover. The radical and the unique heme intermediates persist in wild-type KatG only during the time course of turnover of excess H2O2 (1000-fold or more). Mutation of Met255, Tyr229, or Trp107, which have covalently linked side chains in a unique distal side adduct (MYW) in wild-type KatG, abolishes this radical and the catalase activity. The D-band EPR spectrum of the radical exhibits a rhombic g tensor with dual gx values (2.00550 and 2.00606) and unique gy (2.00344) and gz values (2.00186) similar to but not typical of native tyrosyl radicals. Density functional theory calculations based on a model of an MYW adduct radical built from x-ray coordinates predict experimentally observed hyperfine interactions and a shift in g values away from the native tyrosyl radical. A catalytic role for an MYW adduct radical in the catalase mechanism of KatG is proposed.
Figures



Similar articles
-
Role of the oxyferrous heme intermediate and distal side adduct radical in the catalase activity of Mycobacterium tuberculosis KatG revealed by the W107F mutant.J Biol Chem. 2009 Mar 13;284(11):7030-7. doi: 10.1074/jbc.M808107200. Epub 2009 Jan 12. J Biol Chem. 2009. PMID: 19139098 Free PMC article.
-
Specific function of the Met-Tyr-Trp adduct radical and residues Arg-418 and Asp-137 in the atypical catalase reaction of catalase-peroxidase KatG.J Biol Chem. 2012 Oct 26;287(44):37057-65. doi: 10.1074/jbc.M112.401208. Epub 2012 Aug 23. J Biol Chem. 2012. PMID: 22918833 Free PMC article.
-
Rapid formation of compound II and a tyrosyl radical in the Y229F mutant of Mycobacterium tuberculosis catalase-peroxidase disrupts catalase but not peroxidase function.J Biol Chem. 2003 Nov 7;278(45):44121-7. doi: 10.1074/jbc.M304757200. Epub 2003 Aug 27. J Biol Chem. 2003. PMID: 12944408
-
Catalase in peroxidase clothing: Interdependent cooperation of two cofactors in the catalytic versatility of KatG.Arch Biochem Biophys. 2014 Feb 15;544:27-39. doi: 10.1016/j.abb.2013.11.007. Epub 2013 Nov 23. Arch Biochem Biophys. 2014. PMID: 24280274 Review.
-
Mechanisms of catalase activity of heme peroxidases.Arch Biochem Biophys. 2010 Aug 1;500(1):74-81. doi: 10.1016/j.abb.2010.04.018. Epub 2010 Apr 29. Arch Biochem Biophys. 2010. PMID: 20434429 Review.
Cited by
-
Co-crystallization of N'-benzyl-idene-pyridine-4-carbohydrazide and benzoic acid via autoxidation of benzaldehyde.Acta Crystallogr E Crystallogr Commun. 2023 Jul 4;79(Pt 8):682-685. doi: 10.1107/S2056989023005698. eCollection 2023 Jul 1. Acta Crystallogr E Crystallogr Commun. 2023. PMID: 37601390 Free PMC article.
-
Isoniazid causes heart looping disorder in zebrafish embryos by the induction of oxidative stress.BMC Pharmacol Toxicol. 2020 Mar 12;21(1):22. doi: 10.1186/s40360-020-0399-2. BMC Pharmacol Toxicol. 2020. PMID: 32178728 Free PMC article.
-
Newly Developed Prodrugs and Prodrugs in Development; an Insight of the Recent Years.Molecules. 2020 Feb 17;25(4):884. doi: 10.3390/molecules25040884. Molecules. 2020. PMID: 32079289 Free PMC article. Review.
-
Properties of Site-Specifically Incorporated 3-Aminotyrosine in Proteins To Study Redox-Active Tyrosines: Escherichia coli Ribonucleotide Reductase as a Paradigm.Biochemistry. 2018 Jun 19;57(24):3402-3415. doi: 10.1021/acs.biochem.8b00160. Epub 2018 Apr 17. Biochemistry. 2018. PMID: 29630358 Free PMC article.
-
Mutual synergy between catalase and peroxidase activities of the bifunctional enzyme KatG is facilitated by electron hole-hopping within the enzyme.J Biol Chem. 2017 Nov 10;292(45):18408-18421. doi: 10.1074/jbc.M117.791202. Epub 2017 Sep 27. J Biol Chem. 2017. PMID: 28972181 Free PMC article.
References
-
- Collins, D. M. (1996) Trends Microbiol. 4 426-430 - PubMed
-
- Pym, A. S., Domenech, P., Honore, N., Song, J., Deretic, V., and Cole, S. T. (2001) Mol. Microbiol. 40 879-889 - PubMed
-
- Johnsson, K., King, D. S., and Schultz, P. G. (1995) J. Am. Chem. Soc. 117 5009-5010
-
- Yu, S., Girotto, S., Lee, C., and Magliozzo, R. S. (2003) J. Biol. Chem. 278 14769-14775 - PubMed
-
- Pym, A. S., and Cole, S. T. (2002) in Bacterial Resistance to Antimicrobials: Mechanisms, Genetics, Medical Practice and Public Health (Wax, R., Lewis, K., Salyers, A., and Taber, H., eds) pp. 355-403, Marcel Dekker, Inc., New York
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


