Inhibition of tumour necrosis factor and IL-17 production by leflunomide involves the JAK/STAT pathway
- PMID: 18957484
- PMCID: PMC2946060
- DOI: 10.1136/ard.2008.096743
Inhibition of tumour necrosis factor and IL-17 production by leflunomide involves the JAK/STAT pathway
Abstract
Objective: To study the effects of different disease-modifying antirheumatic drugs (DMARD) on different events mediated by IL-15-activated lymphocytes.
Methods: Peripheral blood lymphocytes (PBL) were isolated from healthy donors and activated with IL-15 after exposure to different DMARD: leflunomide, cyclosporin A, methotrexate, mycophenolic acid, FK-506, sulphasalazine and sodium aurothiomalate. The expression of different surface molecules on the PBL was then determined by flow cytometry. Cells were also co-cultured with the monocytic cell line THP-1 and the tumour necrosis factor (TNF) concentration in the supernatant was measured after 24 h using an immunoenzyme assay. The effect of the aforementioned drugs on IL-17 production by IL-15-activated PBL was also studied.
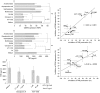
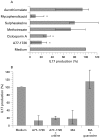
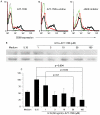
Results: Treatment of PBL with leflunomide, cyclosporin A and FK-506 inhibited the IL-15-induced expression of both CD54 and CD69 by PBL, as well as TNF production in co-cultures of activated PBL and THP-1 cells. The downregulation of CD54 and CD69 in PBL was correlated with the inhibition of TNF production. Likewise, leflunomide, cyclosporin A and FK-506 all inhibited IL-17 production in IL-15-activated PBL. Interestingly, the effect of leflunomide was not reverted by the presence of uridine in the medium. In addition, leflunomide inhibited the phosphorylation of STAT6 in vitro.
Conclusion: Inhibition of the JAK/STAT pathway may represent an additional effect of leflunomide in chronic polyarthritis because it impairs certain events that control proinflammatory TNF and IL-17 cytokine production.
Conflict of interest statement
Figures
Similar articles
-
Regulation of IL-15-stimulated TNF-alpha production by rolipram.J Immunol. 1999 Sep 1;163(5):2836-43. J Immunol. 1999. PMID: 10453029
-
Inhibition of JAK3 and STAT6 tyrosine phosphorylation by the immunosuppressive drug leflunomide leads to a block in IgG1 production.J Immunol. 1998 Feb 15;160(4):1581-8. J Immunol. 1998. PMID: 9469413
-
IL-2 activation of STAT5 enhances production of IL-10 from human cytotoxic regulatory T cells, HOZOT.Exp Hematol. 2008 Feb;36(2):181-92. doi: 10.1016/j.exphem.2007.09.010. Epub 2007 Nov 26. Exp Hematol. 2008. PMID: 18023521
-
Production of tumor necrosis factor-alpha and interferon-gamma from human peripheral blood lymphocytes by MGN-3, a modified arabinoxylan from rice bran, and its synergy with interleukin-2 in vitro.Cancer Detect Prev. 2000;24(4):314-24. Cancer Detect Prev. 2000. PMID: 11059563
-
Tumor necrosis factor-alpha facilitates induction of CD80 (B7-1) and CD54 on human B cells by activated T cells: complex regulation by IL-4, IL-10, and CD40L.Cell Immunol. 1995 Apr 1;161(2):226-35. doi: 10.1006/cimm.1995.1031. Cell Immunol. 1995. PMID: 7535196
Cited by
-
Antirheumatic drug leflunomide attenuates atherosclerosis by regulating lipid metabolism and endothelial dysfunction via DHODH/AMPK signaling pathway.Int J Biol Sci. 2024 Jul 2;20(10):3725-3741. doi: 10.7150/ijbs.93465. eCollection 2024. Int J Biol Sci. 2024. PMID: 39113703 Free PMC article.
-
Effectiveness of methotrexate and leflunomide as corticoid-sparing drugs in patients with polymyalgia rheumatica.Rheumatol Adv Pract. 2024 Mar 21;8(2):rkae033. doi: 10.1093/rap/rkae033. eCollection 2024. Rheumatol Adv Pract. 2024. PMID: 38560643 Free PMC article.
-
Molecular and neuroimmune pharmacology of S1P receptor modulators and other disease-modifying therapies for multiple sclerosis.Pharmacol Ther. 2023 Jun;246:108432. doi: 10.1016/j.pharmthera.2023.108432. Epub 2023 May 4. Pharmacol Ther. 2023. PMID: 37149155 Free PMC article. Review.
-
Plasma interleukin-23 and circulating IL-17A+IFNγ+ ex-Th17 cells predict opposing outcomes of anti-TNF therapy in rheumatoid arthritis.Arthritis Res Ther. 2022 Feb 26;24(1):57. doi: 10.1186/s13075-022-02748-3. Arthritis Res Ther. 2022. PMID: 35219333 Free PMC article.
-
Cell-Based Double-Screening Method to Identify a Reliable Candidate for Osteogenesis-Targeting Compounds.Biomedicines. 2022 Feb 11;10(2):426. doi: 10.3390/biomedicines10020426. Biomedicines. 2022. PMID: 35203635 Free PMC article.
References
-
- Choy E, Panayi G. Mechanisms of action of second-line agents and choice of drugs in combination therapy. Clin Exp Rheumatol 1999;17(Suppl 18):S20–8 - PubMed
-
- Vey E, Zhang JH, Dayer JM. IFN-gamma and 1,25(OH)2D3 induce on THP-1 cells distinct patterns of cell surface antigen expression, cytokine production, and responsiveness to contact with activated T cells. J Immunol 1992;149:2040–6 - PubMed
-
- Isler P, Vey E, Zhang JH, et al. Cell surface glycoproteins expressed on activated human T cells induce production of interleukin-1 beta by monocytic cells: a possible role of CD69. Eur Cytokine Netw 1993;4:15–23 - PubMed
-
- Vey E, Burger D, Dayer JM. Expression and cleavage of tumor necrosis factor-alpha and tumor necrosis factor receptors by human monocytic cell lines upon direct contact with stimulated T cells. Eur J Immunol 1996;26:2404–9 - PubMed
-
- McInnes IB, Leung BP, Sturrock RD, et al. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis [see comments]. Nat Med 1997;3:189–95 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

