The chemokine CXCL1/growth related oncogene increases sodium currents and neuronal excitability in small diameter sensory neurons
- PMID: 18816377
- PMCID: PMC2562993
- DOI: 10.1186/1744-8069-4-38
The chemokine CXCL1/growth related oncogene increases sodium currents and neuronal excitability in small diameter sensory neurons
Abstract
Background: Altered Na+ channel expression, enhanced excitability, and spontaneous activity occur in nerve-injury and inflammatory models of pathological pain, through poorly understood mechanisms. The cytokine GRO/KC (growth related oncogene; CXCL1) shows strong, rapid upregulation in dorsal root ganglion in both nerve injury and inflammatory models. Neurons and glia express its receptor (CXCR2). CXCL1 has well-known effects on immune cells, but little is known about its direct effects on neurons.
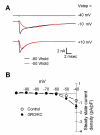
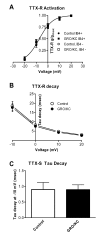
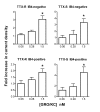
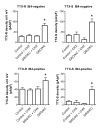
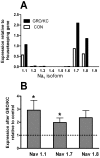
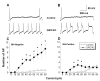
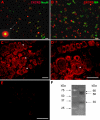
Results: We report that GRO/KC incubation (1.5 nM, overnight) caused marked upregulation of Na+ currents in acutely isolated small diameter rat (adult) sensory neurons in vitro. In both IB4-positive and IB4-negative sensory neurons, TTX-resistant and TTX-sensitive currents increased 2- to 4 fold, without altered voltage dependence or kinetic changes. These effects required long exposures, and were completely blocked by co-incubation with protein synthesis inhibitor cycloheximide. Amplification of cDNA from the neuronal cultures showed that 3 Na channel isoforms were predominant both before and after GRO/KC treatment (Nav 1.1, 1.7, and 1.8). TTX-sensitive isoforms 1.1 and 1.7 significantly increased 2 - 3 fold after GRO/KC incubation, while 1.8 showed a trend towards increased expression. Current clamp experiments showed that GRO/KC caused a marked increase in excitability, including resting potential depolarization, decreased rheobase, and lower action potential threshold. Neurons acquired a striking ability to fire repetitively; IB4-positive cells also showed marked broadening of action potentials. Immunohistochemical labelling confirmed that the CXCR2 receptor was present in most neurons both in dissociated cells and in DRG sections, as previously shown for neurons in the CNS.
Conclusion: Many studies on the role of chemokines in pain conditions have focused on their rapid and indirect effects on neurons, via release of inflammatory mediators from immune and glial cells. Our study suggests that GRO/KC may also have important pro-nociceptive effects via its direct actions on sensory neurons, and may induce long-term changes that involve protein synthesis.
Figures

Similar articles
-
NF-kappaB mediated enhancement of potassium currents by the chemokine CXCL1/growth related oncogene in small diameter rat sensory neurons.Mol Pain. 2009 May 28;5:26. doi: 10.1186/1744-8069-5-26. Mol Pain. 2009. PMID: 19476648 Free PMC article.
-
Increased function of the TRPV1 channel in small sensory neurons after local inflammation or in vitro exposure to the pro-inflammatory cytokine GRO/KC.Neurosci Bull. 2012 Apr;28(2):155-64. doi: 10.1007/s12264-012-1208-8. Neurosci Bull. 2012. PMID: 22466126 Free PMC article.
-
Tetrodotoxin-sensitive and -resistant Na+ channel currents in subsets of small sensory neurons of rats.Brain Res. 2004 Dec 17;1029(2):251-8. doi: 10.1016/j.brainres.2004.09.051. Brain Res. 2004. PMID: 15542080
-
Regulation/modulation of sensory neuron sodium channels.Handb Exp Pharmacol. 2014;221:111-35. doi: 10.1007/978-3-642-41588-3_6. Handb Exp Pharmacol. 2014. PMID: 24737234 Review.
-
New insights into ADAMs regulation of the GRO-α/CXCR2 system: focus on Sjögren's syndrome.Int Rev Immunol. 2015;34(6):486-99. doi: 10.3109/08830185.2014.975892. Epub 2014 Nov 11. Int Rev Immunol. 2015. PMID: 25386842 Review.
Cited by
-
CXCL5 activates CXCR2 in nociceptive sensory neurons to drive joint pain and inflammation in experimental gouty arthritis.Nat Commun. 2024 Apr 16;15(1):3263. doi: 10.1038/s41467-024-47640-7. Nat Commun. 2024. PMID: 38627393 Free PMC article.
-
Microglial knockdown does not affect acute withdrawal but delays analgesic tolerance from oxycodone in male and female C57BL/6J mice.Adv Drug Alcohol Res. 2022 Dec 16;2:10848. doi: 10.3389/adar.2022.10848. eCollection 2022. Adv Drug Alcohol Res. 2022. PMID: 38390615 Free PMC article.
-
Differential expression of slow and fast-repriming tetrodotoxin-sensitive sodium currents in dorsal root ganglion neurons.Front Mol Neurosci. 2024 Jan 11;16:1336664. doi: 10.3389/fnmol.2023.1336664. eCollection 2023. Front Mol Neurosci. 2024. PMID: 38273939 Free PMC article.
-
Afferent neurons of the kidney with impaired firing pattern in inflammation - role of sodium currents?Pflugers Arch. 2023 Nov;475(11):1329-1342. doi: 10.1007/s00424-023-02852-6. Epub 2023 Sep 6. Pflugers Arch. 2023. PMID: 37672108 Free PMC article.
-
Chemokine CCL7 mediates trigeminal neuropathic pain via CCR2/CCR3-ERK pathway in the trigeminal ganglion of mice.Mol Pain. 2023 Jan-Dec;19:17448069231169373. doi: 10.1177/17448069231169373. Mol Pain. 2023. PMID: 36998150 Free PMC article.
References
-
- Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol. 2003;521:1–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases


