Voltage-gated Na+ channel beta1 subunit-mediated neurite outgrowth requires Fyn kinase and contributes to postnatal CNS development in vivo
- PMID: 18354028
- PMCID: PMC6670697
- DOI: 10.1523/JNEUROSCI.5446-07.2008
Voltage-gated Na+ channel beta1 subunit-mediated neurite outgrowth requires Fyn kinase and contributes to postnatal CNS development in vivo
Abstract
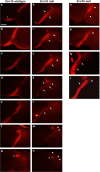
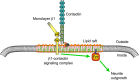
Voltage-gated Na(+) channel beta1 subunits are multifunctional, participating in channel modulation and cell adhesion in vitro. We previously demonstrated that beta1 promotes neurite outgrowth of cultured cerebellar granule neurons (CGNs) via homophilic adhesion. Both lipid raft-associated kinases and nonraft fibroblast growth factor (FGF) receptors are implicated in cell adhesion molecule-mediated neurite extension. In the present study, we reveal that beta1-mediated neurite outgrowth is abrogated in Fyn and contactin (Cntn) null CGNs. beta1 protein levels are unchanged in Fyn null brains, whereas levels are significantly reduced in Cntn null brain lysates. FGF or EGF (epidermal growth factor) receptor kinase inhibitors have no effect on beta1-mediated neurite extension. These results suggest that beta1-mediated neurite outgrowth occurs through a lipid raft signaling mechanism that requires the presence of both fyn kinase and contactin. In vivo, Scn1b null mice show defective CGN axon extension and fasciculation indicating that beta1 plays a role in cerebellar microorganization. In addition, we find that axonal pathfinding and fasciculation are abnormal in corticospinal tracts of Scn1b null mice consistent with the suggestion that beta1 may have widespread effects on postnatal neuronal development. These data are the first to demonstrate a cell-adhesive role for beta1 in vivo. We conclude that voltage-gated Na(+) channel beta1 subunits signal via multiple pathways on multiple timescales and play important roles in the postnatal development of the CNS.
Figures
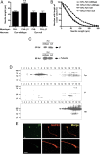
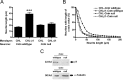
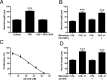

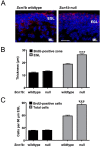
Similar articles
-
Functional reciprocity between Na+ channel Nav1.6 and beta1 subunits in the coordinated regulation of excitability and neurite outgrowth.Proc Natl Acad Sci U S A. 2010 Feb 2;107(5):2283-8. doi: 10.1073/pnas.0909434107. Epub 2010 Jan 19. Proc Natl Acad Sci U S A. 2010. PMID: 20133873 Free PMC article.
-
The sodium channel β1 subunit mediates outgrowth of neurite-like processes on breast cancer cells and promotes tumour growth and metastasis.Int J Cancer. 2014 Nov 15;135(10):2338-51. doi: 10.1002/ijc.28890. Epub 2014 Apr 26. Int J Cancer. 2014. PMID: 24729314 Free PMC article.
-
β1-C121W Is Down But Not Out: Epilepsy-Associated Scn1b-C121W Results in a Deleterious Gain-of-Function.J Neurosci. 2016 Jun 8;36(23):6213-24. doi: 10.1523/JNEUROSCI.0405-16.2016. J Neurosci. 2016. PMID: 27277800 Free PMC article.
-
Electrophysiology and beyond: multiple roles of Na+ channel β subunits in development and disease.Neurosci Lett. 2010 Dec 10;486(2):53-9. doi: 10.1016/j.neulet.2010.06.050. Epub 2010 Jun 23. Neurosci Lett. 2010. PMID: 20600605 Free PMC article. Review.
-
An emerging role for voltage-gated Na+ channels in cellular migration: regulation of central nervous system development and potentiation of invasive cancers.Neuroscientist. 2008 Dec;14(6):571-83. doi: 10.1177/1073858408320293. Epub 2008 Oct 20. Neuroscientist. 2008. PMID: 18940784 Free PMC article. Review.
Cited by
-
Complex Synaptic and Intrinsic Interactions Disrupt Input/Output Functions in the Hippocampus of Scn1b Knock-Out Mice.J Neurosci. 2023 Dec 6;43(49):8562-8577. doi: 10.1523/JNEUROSCI.0786-23.2023. J Neurosci. 2023. PMID: 37845033 Free PMC article.
-
Voltage-gated sodium channels: from roles and mechanisms in the metastatic cell behavior to clinical potential as therapeutic targets.Front Pharmacol. 2023 Jun 30;14:1206136. doi: 10.3389/fphar.2023.1206136. eCollection 2023. Front Pharmacol. 2023. PMID: 37456756 Free PMC article. Review.
-
Sodium channels and the ionic microenvironment of breast tumours.J Physiol. 2023 May;601(9):1543-1553. doi: 10.1113/JP282306. Epub 2022 Oct 21. J Physiol. 2023. PMID: 36183245 Free PMC article.
-
Neonatal Scn1b-null mice have sinoatrial node dysfunction, altered atrial structure, and atrial fibrillation.JCI Insight. 2022 May 23;7(10):e152050. doi: 10.1172/jci.insight.152050. JCI Insight. 2022. PMID: 35603785 Free PMC article.
-
Fyn Kinase-Mediated PKCδ Y311 Phosphorylation Induces Dopaminergic Degeneration in Cell Culture and Animal Models: Implications for the Identification of a New Pharmacological Target for Parkinson's Disease.Front Pharmacol. 2021 Apr 28;12:631375. doi: 10.3389/fphar.2021.631375. eCollection 2021. Front Pharmacol. 2021. PMID: 33995031 Free PMC article.
References
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. 3. Maturation of the components of the granular layer. J Comp Neurol. 1972;145:465–513. - PubMed
-
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. - PubMed
-
- Audenaert D, Claes L, Ceulemans B, Lofgren A, Van Broeckhoven C, De Jonghe P. A deletion in SCN1B is associated with febrile seizures and early-onset absence epilepsy. Neurology. 2003;61:854–856. - PubMed
-
- Bailly Y, Kyriakopoulou K, Delhaye-Bouchaud N, Mariani J, Karagogeos D. Cerebellar granule cell differentiation in mutant and X-irradiated rodents revealed by the neural adhesion molecule TAG-1. J Comp Neurol. 1996;369:150–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

