Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence
- PMID: 18322084
- PMCID: PMC4028226
- DOI: 10.1523/JNEUROSCI.5064-07.2008
Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence
Abstract
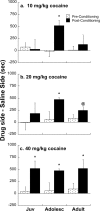
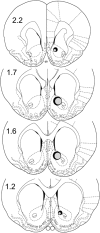
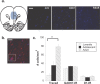
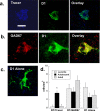
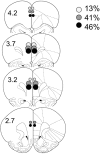
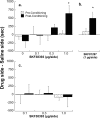
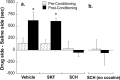
Adolescence is a transitional period during development that is associated with a greater likelihood of addiction to drugs than any other age. In the prefrontal cortex (PFC), D(1) dopamine receptors mediate motivational salience attribution, which plays a role in addiction. Here, we investigated the relationship of age-related D(1) dopamine receptor expression in the PFC with the maturation of cocaine place conditioning. Confocal microscopy revealed that retrogradely traced cortical output neurons to the nucleus accumbens express higher levels of D(1) receptors during adolescence compared with younger and older ages. D(1) expression does not change on GABAergic interneurons across age. Adolescent differences in D(1) expression occur independently of cortical-accumbens connectivity, which proliferates through adulthood. Behaviorally, adolescent rats are more sensitive to cocaine place conditioning than younger and older rats. However, microinjections of the D(1) antagonist SCH23390 into the PFC blocked adolescent place preferences, whereas microinjections of D(1) agonists dose-dependently increased preferences for cocaine-associated environments previously not preferred by juveniles. These results suggest that the heightened expression of D(1) receptors on cortical-accumbens projections may help explain increased sensitivity to environmental events and addictive behaviors during adolescence, whereas the paucity of D(1)-expressing projections may reduce risk in juveniles.
Figures
Similar articles
-
Enhancing the salience of dullness: behavioral and pharmacological strategies to facilitate extinction of drug-cue associations in adolescent rats.Neuroscience. 2010 Aug 25;169(2):628-36. doi: 10.1016/j.neuroscience.2010.05.063. Neuroscience. 2010. PMID: 20639130 Free PMC article.
-
Extinction and reinstatement to cocaine-associated cues in male and female juvenile rats and the role of D1 dopamine receptor.Neuropharmacology. 2015 Aug;95:22-8. doi: 10.1016/j.neuropharm.2015.02.017. Epub 2015 Mar 4. Neuropharmacology. 2015. PMID: 25749358 Free PMC article.
-
Viral over-expression of D1 dopamine receptors in the prefrontal cortex increase high-risk behaviors in adults: comparison with adolescents.Psychopharmacology (Berl). 2014 Apr;231(8):1615-26. doi: 10.1007/s00213-013-3399-8. Epub 2014 Jan 10. Psychopharmacology (Berl). 2014. PMID: 24408208 Free PMC article.
-
Sex-dependent changes in ADHD-like behaviors in juvenile rats following cortical dopamine depletion.Behav Brain Res. 2014 Aug 15;270:357-63. doi: 10.1016/j.bbr.2014.05.024. Epub 2014 May 23. Behav Brain Res. 2014. PMID: 24861711 Free PMC article.
-
Novelty preferences and cocaine-associated cues influence regions associated with the salience network in juvenile female rats.Pharmacol Biochem Behav. 2021 Apr;203:173117. doi: 10.1016/j.pbb.2021.173117. Epub 2021 Feb 6. Pharmacol Biochem Behav. 2021. PMID: 33561479
Cited by
-
Dopaminergic Perturbation in the Aetiology of Neurodevelopmental Disorders.Mol Neurobiol. 2024 Aug 7. doi: 10.1007/s12035-024-04418-8. Online ahead of print. Mol Neurobiol. 2024. PMID: 39110391 Review.
-
Increasing CB2 Receptor Activity after Early Life Stress Prevents Depressive Behavior in Female Rats.Biomolecules. 2024 Apr 10;14(4):464. doi: 10.3390/biom14040464. Biomolecules. 2024. PMID: 38672480 Free PMC article.
-
An enriched environment ameliorates the reduction of parvalbumin-positive interneurons in the medial prefrontal cortex caused by maternal separation early in life.Front Neurosci. 2024 Jan 16;17:1308368. doi: 10.3389/fnins.2023.1308368. eCollection 2023. Front Neurosci. 2024. PMID: 38292903 Free PMC article.
-
Cocaine reward and reinstatement in adolescent versus adult rodents.Front Behav Neurosci. 2024 Jan 5;17:1278263. doi: 10.3389/fnbeh.2023.1278263. eCollection 2023. Front Behav Neurosci. 2024. PMID: 38249124 Free PMC article. Review.
-
Brexpiprazole in patients with schizophrenia with or without substance use disorder: an observational study.Front Psychiatry. 2023 Dec 4;14:1321233. doi: 10.3389/fpsyt.2023.1321233. eCollection 2023. Front Psychiatry. 2023. PMID: 38111619 Free PMC article.
References
-
- Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, Neisewander JL. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2002;159:284–293. - PubMed
-
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. - PubMed
-
- Andersen SL, LeBlanc CJ, Lyss PJ. Maturational increases in c-fos expression in the ascending dopamine systems. Synapse. 2001;41:345–350. - PubMed
-
- Andersen SL, Thompson A, Krenzel E, Teicher M. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002a;27:683–691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

