Kinetic isotope effects for alkaline phosphatase reactions: implications for the role of active-site metal ions in catalysis
- PMID: 17630738
- PMCID: PMC3171187
- DOI: 10.1021/ja072196+
Kinetic isotope effects for alkaline phosphatase reactions: implications for the role of active-site metal ions in catalysis
Abstract
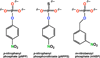
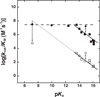
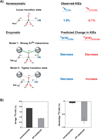
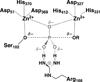
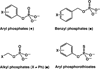
Enzyme-catalyzed phosphoryl transfer reactions have frequently been suggested to proceed through transition states that are altered from their solution counterparts, with the alterations presumably arising from interactions with active-site functional groups. In particular, the phosphate monoester hydrolysis reaction catalyzed by Escherichia coli alkaline phosphatase (AP) has been the subject of intensive scrutiny. Recent linear free energy relationship (LFER) studies suggest that AP catalyzes phosphate monoester hydrolysis through a loose transition state, similar to that in solution. To gain further insight into the nature of the transition state and active-site interactions, we have determined kinetic isotope effects (KIEs) for AP-catalyzed hydrolysis reactions with several phosphate monoester substrates. The LFER and KIE data together provide a consistent picture for the nature of the transition state for AP-catalyzed phosphate monoester hydrolysis and support previous models suggesting that the enzymatic transition state is similar to that in solution. Moreover, the KIE data provides unique information regarding specific interactions between the transition state and the active-site Zn2+ ions. These results provide strong support for a model in which electrostatic interactions between the bimetallo Zn2+ site and a nonbridging phosphate ester oxygen atom make a significant contribution to the large rate enhancement observed for AP-catalyzed phosphate monoester hydrolysis.
Figures
Similar articles
-
Probing the origin of the compromised catalysis of E. coli alkaline phosphatase in its promiscuous sulfatase reaction.J Am Chem Soc. 2007 May 2;129(17):5760-5. doi: 10.1021/ja069111+. Epub 2007 Apr 6. J Am Chem Soc. 2007. PMID: 17411045 Free PMC article.
-
Alkaline phosphatase mono- and diesterase reactions: comparative transition state analysis.J Am Chem Soc. 2006 Feb 1;128(4):1293-303. doi: 10.1021/ja056528r. J Am Chem Soc. 2006. PMID: 16433548 Free PMC article.
-
Functional interrelationships in the alkaline phosphatase superfamily: phosphodiesterase activity of Escherichia coli alkaline phosphatase.Biochemistry. 2001 May 15;40(19):5691-9. doi: 10.1021/bi0028892. Biochemistry. 2001. PMID: 11341834
-
Structure and mechanism of alkaline phosphatase.Annu Rev Biophys Biomol Struct. 1992;21:441-83. doi: 10.1146/annurev.bb.21.060192.002301. Annu Rev Biophys Biomol Struct. 1992. PMID: 1525473 Review.
-
The mechanism of the alkaline phosphatase reaction: insights from NMR, crystallography and site-specific mutagenesis.FEBS Lett. 1999 Nov 26;462(1-2):7-11. doi: 10.1016/s0014-5793(99)01448-9. FEBS Lett. 1999. PMID: 10580082 Review.
Cited by
-
What Does the Brønsted Slope Measure in the Phosphoryl Transfer Transition State?ACS Catal. 2020 Dec 4;10(23):13932-13945. doi: 10.1021/acscatal.0c03764. Epub 2020 Nov 16. ACS Catal. 2020. PMID: 34567831 Free PMC article.
-
Differences in the Nature of the Phosphoryl Transfer Transition State in Protein Phosphatase 1 and Alkaline Phosphatase: Insights from QM Cluster Models.J Phys Chem B. 2020 Oct 22;124(42):9371-9384. doi: 10.1021/acs.jpcb.0c07863. Epub 2020 Oct 8. J Phys Chem B. 2020. PMID: 33030898 Free PMC article.
-
Mechanistic Studies of Homo- and Heterodinuclear Zinc Phosphoesterase Mimics: What Has Been Learned?Front Chem. 2019 Feb 21;7:82. doi: 10.3389/fchem.2019.00082. eCollection 2019. Front Chem. 2019. PMID: 30847339 Free PMC article. Review.
-
Transition-State Interactions in a Promiscuous Enzyme: Sulfate and Phosphate Monoester Hydrolysis by Pseudomonas aeruginosa Arylsulfatase.Biochemistry. 2019 Mar 12;58(10):1363-1378. doi: 10.1021/acs.biochem.8b00996. Epub 2019 Feb 27. Biochemistry. 2019. PMID: 30810299 Free PMC article.
-
Transition State Analysis of the Reaction Catalyzed by the Phosphotriesterase from Sphingobium sp. TCM1.Biochemistry. 2019 Mar 5;58(9):1246-1259. doi: 10.1021/acs.biochem.9b00041. Epub 2019 Feb 19. Biochemistry. 2019. PMID: 30730705 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources


