A Bayesian perceptual model replicates the cutaneous rabbit and other tactile spatiotemporal illusions
- PMID: 17389923
- PMCID: PMC1828626
- DOI: 10.1371/journal.pone.0000333
A Bayesian perceptual model replicates the cutaneous rabbit and other tactile spatiotemporal illusions
Abstract
Background: When brief stimuli contact the skin in rapid succession at two or more locations, perception strikingly shrinks the intervening distance, and expands the elapsed time, between consecutive events. The origins of these perceptual space-time distortions are unknown.
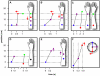
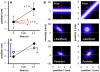
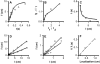
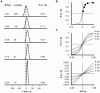
Methodology/principal findings: Here I show that these illusory effects, which I term perceptual length contraction and time dilation, are emergent properties of a Bayesian observer model that incorporates prior expectation for speed. Rapidly moving stimuli violate expectation, provoking perceptual length contraction and time dilation. The Bayesian observer replicates the cutaneous rabbit illusion, the tau effect, the kappa effect, and other spatiotemporal illusions. Additionally, it shows realistic tactile temporal order judgment and spatial attention effects.
Conclusions/significance: The remarkable explanatory power of this simple model supports the hypothesis, first proposed by Helmholtz, that the brain biases perception in favor of expectation. Specifically, the results suggest that the brain automatically incorporates prior expectation for speed in order to overcome spatial and temporal imprecision inherent in the sensorineural signal.
Conflict of interest statement
Figures
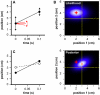
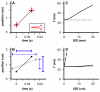

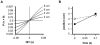
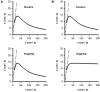
Similar articles
-
Prediction, postdiction, and perceptual length contraction: a bayesian low-speed prior captures the cutaneous rabbit and related illusions.Front Psychol. 2013 May 10;4:221. doi: 10.3389/fpsyg.2013.00221. eCollection 2013. Front Psychol. 2013. PMID: 23675360 Free PMC article.
-
Tactile length contraction as Bayesian inference.J Neurophysiol. 2016 Aug 1;116(2):369-79. doi: 10.1152/jn.00029.2016. Epub 2016 Apr 27. J Neurophysiol. 2016. PMID: 27121574 Free PMC article.
-
Illusory tactile movement crosses arms and legs and is coded in external space.Cortex. 2022 Apr;149:202-225. doi: 10.1016/j.cortex.2022.01.014. Epub 2022 Feb 5. Cortex. 2022. PMID: 35272063
-
Perception, illusions and Bayesian inference.Psychopathology. 2015;48(4):217-21. doi: 10.1159/000437271. Epub 2015 Aug 8. Psychopathology. 2015. PMID: 26279057 Review.
-
[Temporal order judgment in the brain].Brain Nerve. 2008 Mar;60(3):263-72. Brain Nerve. 2008. PMID: 18402074 Review. Japanese.
Cited by
-
Spatiotemporal motion features resulting from tactile interface layouts influence tactile speed perception.iScience. 2024 Aug 30;27(9):110803. doi: 10.1016/j.isci.2024.110803. eCollection 2024 Sep 20. iScience. 2024. PMID: 39310745 Free PMC article.
-
Same principle, but different computations in representing time and space.Front Neurosci. 2024 May 7;18:1387641. doi: 10.3389/fnins.2024.1387641. eCollection 2024. Front Neurosci. 2024. PMID: 38774789 Free PMC article.
-
Tactile shape discrimination for moving stimuli.Sci Rep. 2024 Apr 15;14(1):8707. doi: 10.1038/s41598-024-58509-6. Sci Rep. 2024. PMID: 38622201 Free PMC article.
-
Modality-Independent Effect of Gravity in Shaping the Internal Representation of 3D Space for Visual and Haptic Object Perception.J Neurosci. 2024 Mar 27;44(13):e2457202023. doi: 10.1523/JNEUROSCI.2457-20.2023. J Neurosci. 2024. PMID: 38267257 Free PMC article.
-
Thermal illusions for thermal displays: a review.Front Hum Neurosci. 2023 Dec 5;17:1278894. doi: 10.3389/fnhum.2023.1278894. eCollection 2023. Front Hum Neurosci. 2023. PMID: 38116235 Free PMC article. Review.
References
-
- Wassle H, Grunert U, Rohrenbeck J, Boycott BB. Retinal ganglion cell density and cortical magnification factor in the primate. Vision Res. 1990;30:1897–1911. - PubMed
-
- Johnson KO, Phillips JR. Tactile spatial resolution. I. Two-point discrimination, gap detection, grating resolution, and letter recognition. J Neurophysiol. 1981;46:1177–1192. - PubMed
-
- Weinstein S. The skin senses : proceedings. Springfield, Ill.: Thomas; 1968. Intensive and extensive aspects of tactile sensitivity as a function of body part, sex, and laterality. pp. 195–222.
MeSH terms
LinkOut - more resources
Full Text Sources


