Dietary sequestration of defensive steroids in nuchal glands of the Asian snake Rhabdophis tigrinus
- PMID: 17284596
- PMCID: PMC1892995
- DOI: 10.1073/pnas.0610785104
Dietary sequestration of defensive steroids in nuchal glands of the Asian snake Rhabdophis tigrinus
Abstract
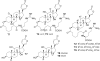
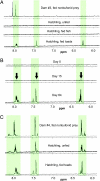
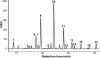
The Asian snake Rhabdophis tigrinus possesses specialized defensive glands on its neck that contain steroidal toxins known as bufadienolides. We hypothesized that R. tigrinus does not synthesize these defensive steroids but instead sequesters the toxins from toads it consumes as prey. To test this hypothesis, we conducted chemical analyses on the glandular fluid from snakes collected in toad-free and toad-present localities. We also performed feeding experiments in which hatchling R. tigrinus were reared on controlled diets that either included or lacked toads. We demonstrate that the cardiotonic steroids in the nuchal glands of R. tigrinus are obtained from dietary toads. We further show that mothers containing high levels of bufadienolides can provision their offspring with toxins. Hatchlings had bufadienolides in their nuchal glands only if they were fed toads or were born to a dam with high concentrations of these compounds. Because geographic patterns in the availability of toxic prey are reflected in the chemical composition of the glandular fluid, snakes in toad-free regions are left undefended by steroidal toxins. Our findings confirm that the sequestration of dietary toxins underlies geographic variation in antipredatory behavior in this species and provide a unique example of sequestered defensive compounds in a specialized vertebrate structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Chemical defense of an Asian snake reflects local availability of toxic prey and hatchling diet.J Zool (1987). 2013 Apr;289(4):270-278. doi: 10.1111/jzo.12004. Epub 2012 Dec 17. J Zool (1987). 2013. PMID: 23853424 Free PMC article.
-
Does prey matter? Geographic variation in antipredator responses of hatchlings of a Japanese natricine snake (Rhabdophis tigrinus).J Comp Psychol. 2000 Dec;114(4):408-13. doi: 10.1037/0735-7036.114.4.408. J Comp Psychol. 2000. PMID: 11149545
-
New Insights Into Dietary Toxin Metabolism: Diversity in the Ability of the Natricine Snake Rhabdophis tigrinus to Convert Toad-Derived Bufadienolides.J Chem Ecol. 2021 Nov;47(10-11):915-925. doi: 10.1007/s10886-021-01287-6. Epub 2021 Jul 14. J Chem Ecol. 2021. PMID: 34258693
-
The Development of Toad Toxins as Potential Therapeutic Agents.Toxins (Basel). 2018 Aug 20;10(8):336. doi: 10.3390/toxins10080336. Toxins (Basel). 2018. PMID: 30127299 Free PMC article. Review.
-
Chemistry and the Potential Antiviral, Anticancer, and Anti-Inflammatory Activities of Cardiotonic Steroids Derived from Toads.Molecules. 2022 Oct 5;27(19):6586. doi: 10.3390/molecules27196586. Molecules. 2022. PMID: 36235123 Free PMC article. Review.
Cited by
-
The Amphibian Genomics Consortium: advancing genomic and genetic resources for amphibian research and conservation.BMC Genomics. 2024 Nov 1;25(1):1025. doi: 10.1186/s12864-024-10899-7. BMC Genomics. 2024. PMID: 39487448 Free PMC article. Review.
-
The Amphibian Genomics Consortium: advancing genomic and genetic resources for amphibian research and conservation.bioRxiv [Preprint]. 2024 Oct 3:2024.06.27.601086. doi: 10.1101/2024.06.27.601086. bioRxiv. 2024. Update in: BMC Genomics. 2024 Nov 1;25(1):1025. doi: 10.1186/s12864-024-10899-7. PMID: 39005434 Free PMC article. Updated. Preprint.
-
Where Does All the Poison Go? Investigating Toxicokinetics of Newt (Taricha) Tetrodotoxin (TTX) in Garter Snakes (Thamnophis).J Chem Ecol. 2024 Oct;50(9-10):489-502. doi: 10.1007/s10886-024-01517-7. Epub 2024 Jun 6. J Chem Ecol. 2024. PMID: 38842636
-
3α-Hydroxybufadienolides in Bufo gallbladders: structural insights and biotransformation.Nat Prod Bioprospect. 2024 Mar 4;14(1):19. doi: 10.1007/s13659-024-00442-2. Nat Prod Bioprospect. 2024. PMID: 38436763 Free PMC article.
-
The first record of leucism in the Rhabdophis tigrinus (Boie, 1826) (Squamata, Colubridae) in South Korea.Ecol Evol. 2024 Feb 22;14(2):e11029. doi: 10.1002/ece3.11029. eCollection 2024 Feb. Ecol Evol. 2024. PMID: 38390002 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources


