Organic haze on Titan and the early Earth
- PMID: 17101962
- PMCID: PMC1838702
- DOI: 10.1073/pnas.0608561103
Organic haze on Titan and the early Earth
Abstract
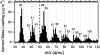
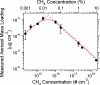
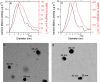
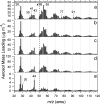
Recent exploration by the Cassini/Huygens mission has stimulated a great deal of interest in Saturn's moon, Titan. One of Titan's most captivating features is the thick organic haze layer surrounding the moon, believed to be formed from photochemistry high in the CH(4)/N(2) atmosphere. It has been suggested that a similar haze layer may have formed on the early Earth. Here we report laboratory experiments that demonstrate the properties of haze likely to form through photochemistry on Titan and early Earth. We have used a deuterium lamp to initiate particle production in these simulated atmospheres from UV photolysis. Using a unique analysis technique, the aerosol mass spectrometer, we have studied the chemical composition, size, and shape of the particles produced as a function of initial trace gas composition. Our results show that the aerosols produced in the laboratory can serve as analogs for the observed haze in Titan's atmosphere. Experiments performed under possible conditions for early Earth suggest a significant optical depth of haze may have dominated the early Earth's atmosphere. Aerosol size measurements are presented, and implications for the haze layer properties are discussed. We estimate that aerosol production on the early Earth may have been on the order of 10(14) g.year(-1) and thus could have served as a primary source of organic material to the surface.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Reduction in haze formation rate on prebiotic Earth in the presence of hydrogen.Astrobiology. 2009 Jun;9(5):447-53. doi: 10.1089/ast.2008.0289. Astrobiology. 2009. PMID: 19566425
-
An overview of the descent and landing of the Huygens probe on Titan.Nature. 2005 Dec 8;438(7069):758-64. doi: 10.1038/nature04347. Epub 2005 Nov 30. Nature. 2005. PMID: 16319826
-
The Impact of Molecular Oxygen on Anion Composition in a Hazy Archean Earth Atmosphere.Astrobiology. 2020 May;20(5):658-669. doi: 10.1089/ast.2019.2145. Epub 2020 Mar 10. Astrobiology. 2020. PMID: 32159384
-
Cassini-Huygens' exploration of the Saturn system: 13 years of discovery.Science. 2019 Jun 14;364(6445):1046-1051. doi: 10.1126/science.aat3760. Science. 2019. PMID: 31197006 Review.
-
Prebiotic-like chemistry on Titan.Chem Soc Rev. 2012 Aug 21;41(16):5380-93. doi: 10.1039/c2cs35014a. Epub 2012 Apr 5. Chem Soc Rev. 2012. PMID: 22481630 Review.
Cited by
-
Reactant Discovery with an Ab Initio Nanoreactor: Exploration of Astrophysical N-Heterocycle Precursors and Formation Pathways.ACS Earth Space Chem. 2024 Aug 9;8(9):1771-1783. doi: 10.1021/acsearthspacechem.4c00120. eCollection 2024 Sep 19. ACS Earth Space Chem. 2024. PMID: 39318708 Free PMC article.
-
The Composition and Chemistry of Titan's Atmosphere.ACS Earth Space Chem. 2024 Feb 29;8(3):406-456. doi: 10.1021/acsearthspacechem.2c00041. eCollection 2024 Mar 21. ACS Earth Space Chem. 2024. PMID: 38533193 Free PMC article. Review.
-
A revised lower estimate of ozone columns during Earth's oxygenated history.R Soc Open Sci. 2022 Jan 5;9(1):211165. doi: 10.1098/rsos.211165. eCollection 2022 Jan. R Soc Open Sci. 2022. PMID: 35070343 Free PMC article.
-
On an EUV Atmospheric Simulation Chamber to Study the Photochemical Processes of Titan's Atmosphere.Sci Rep. 2020 Jun 19;10(1):10009. doi: 10.1038/s41598-020-66950-6. Sci Rep. 2020. PMID: 32561886 Free PMC article.
-
The Archean atmosphere.Sci Adv. 2020 Feb 26;6(9):eaax1420. doi: 10.1126/sciadv.aax1420. eCollection 2020 Feb. Sci Adv. 2020. PMID: 32133393 Free PMC article. Review.
References
-
- Porco CC, Baker E, Barbara J, Beurle K, Brahic A, Burns JA, Charnoz S, Cooper N, Dawson DD, Del Genio AD, et al. Nature. 2005;434:159–168. - PubMed
-
- Waite JH, Niemann H, Yelle RV, Kasprzak WT, Cravens TE, Luhmann JG, McNutt RL, Ip WH, Gell D, De La Haye V, et al. Science. 2005;308:982–986. - PubMed
-
- Flasar FM, Achterberg RK, Conrath BJ, Gierasch PJ, Kunde VG, Nixon CA, Bjoraker GL, Jennings DE, Romani PN, Simon-Miller AA, et al. Science. 2005;308:975–978. - PubMed
-
- Shemansky DE, Stewart AIF, West RA, Esposito LW, Hallett JT, Liu XM. Science. 2005;308:978–982. - PubMed
-
- Israel G, Szopa C, Raulin F, Cabane M, Niemann HB, Atreya SK, Bauer SJ, Brun J-F, Chassefiere E, Coll P, et al. Nature. 2005;438:796–799. - PubMed
LinkOut - more resources
Full Text Sources


