Rivaroxaban (BAY 59-7939)--an oral, direct Factor Xa inhibitor--has no clinically relevant interaction with naproxen
- PMID: 17100983
- PMCID: PMC2203251
- DOI: 10.1111/j.1365-2125.2006.02776.x
Rivaroxaban (BAY 59-7939)--an oral, direct Factor Xa inhibitor--has no clinically relevant interaction with naproxen
Abstract
Aims: Rivaroxaban (BAY 59-7939) is in advanced clinical development for the prevention and treatment of thromboembolic disorders. Frequent co-medications in the patient populations likely to receive rivaroxaban include NSAIDs. This randomized, two-way crossover study, with a naproxen run-in period, was performed to determine whether naproxen influences the tolerability, pharmacodynamics and pharmacokinetics of rivaroxaban.
Methods: Eleven healthy, young males received naproxen 500 mg on two consecutive days, a single dose of rivaroxaban 15 mg, or both.
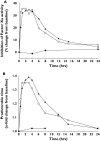
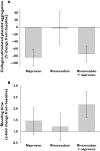
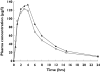
Results: Treatments were well tolerated: adverse events (eight in total), reported by three subjects, were mild and not drug related. Rivaroxaban inhibited Factor Xa activity by 35% and prolonged prothrombin time [by 1.4 times baseline (tb)], activated partial thromboplastin time (1.3 tb) and the HepTest (1.9 tb). Naproxen had no influence on these measures and the combination of rivaroxaban and naproxen did not affect platelet aggregation. Rivaroxaban and naproxen given together significantly increased bleeding time compared with rivaroxaban alone (P = 0.017). However, this difference was small compared with the effect of naproxen given alone, except in one subject. Least squares-means ratios for the AUC and C(max) of rivaroxaban after administration alone and with naproxen were 1.125 [90% confidence interval (CI) 0.995, 1.271] and 1.095 (90% CI 0.905, 1.325), respectively.
Conclusions: There appeared to be no clinically relevant interaction between rivaroxaban and naproxen in healthy subjects, although some individuals may be more sensitive to the combination. Large-scale Phase III clinical studies will be required to confirm whether there is an increased risk of bleeding during treatment with rivaroxaban and concomitant NSAIDs.
Figures
Similar articles
-
Safety, tolerability, pharmacodynamics, and pharmacokinetics of rivaroxaban--an oral, direct factor Xa inhibitor--are not affected by aspirin.J Clin Pharmacol. 2006 Sep;46(9):981-90. doi: 10.1177/0091270006292127. J Clin Pharmacol. 2006. PMID: 16920892 Clinical Trial.
-
Preclinical and clinical characteristics of rivaroxaban: a novel, oral, direct factor Xa inhibitor.Semin Thromb Hemost. 2007 Jul;33(5):515-23. doi: 10.1055/s-2007-982083. Semin Thromb Hemost. 2007. PMID: 17629849 Review.
-
Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59-7939) in healthy subjects.J Clin Pharmacol. 2007 Feb;47(2):218-26. doi: 10.1177/0091270006296058. J Clin Pharmacol. 2007. PMID: 17244773 Clinical Trial.
-
Prevention and treatment of experimental thrombosis in rabbits with rivaroxaban (BAY 597939)--an oral, direct factor Xa inhibitor.Thromb Haemost. 2007 Mar;97(3):471-7. Thromb Haemost. 2007. PMID: 17334516
-
Rivaroxaban: a novel, oral, direct factor Xa inhibitor.Pharmacotherapy. 2009 Feb;29(2):167-81. doi: 10.1592/phco.29.2.167. Pharmacotherapy. 2009. PMID: 19170587 Review.
Cited by
-
Optimization of wet granulation process for manufacturing Rivaroxban generic immediate-release tablets using PBPK modeling and simulations.In Silico Pharmacol. 2024 Aug 22;12(2):77. doi: 10.1007/s40203-024-00249-6. eCollection 2024. In Silico Pharmacol. 2024. PMID: 39184229
-
Investigating Intestinal Transporter Involvement in Rivaroxaban Disposition through Examination of Changes in Absorption.Pharm Res. 2021 May;38(5):795-801. doi: 10.1007/s11095-021-03039-3. Epub 2021 Apr 13. Pharm Res. 2021. PMID: 33847849 Free PMC article.
-
Drug-Drug Interactions Leading to Adverse Drug Reactions with Rivaroxaban: A Systematic Review of the Literature and Analysis of VigiBase.J Pers Med. 2021 Mar 30;11(4):250. doi: 10.3390/jpm11040250. J Pers Med. 2021. PMID: 33808367 Free PMC article. Review.
-
An engineered factor Va prevents bleeding induced by direct-acting oral anticoagulants by different mechanisms.Blood Adv. 2020 Aug 11;4(15):3716-3727. doi: 10.1182/bloodadvances.2020001699. Blood Adv. 2020. PMID: 32777068 Free PMC article.
-
Drug-Drug Interactions with Direct Oral Anticoagulants.Clin Pharmacokinet. 2020 Aug;59(8):967-980. doi: 10.1007/s40262-020-00879-x. Clin Pharmacokinet. 2020. PMID: 32157630 Free PMC article. Review.
References
-
- Paiement GD, Mendelsohn C. The risk of venous thromboembolism in the orthopedic patient: epidemiological and physiological data. Orthopedics. 1997;20(Suppl.):7–9. - PubMed
-
- Ansell J, Bergqvist D. Current options in the prevention of thromboembolic disease. Drugs. 2004;64:1–5. - PubMed
-
- Weitz JI, Bates SM. New anticoagulants. J Thromb Haemost. 2005;3:1843–53. - PubMed
-
- Perzborn E, Strassburger J, Wilmen A, Pohlmann J, Roehrig S, Schlemmer KH, Straub A. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct Factor Xa inhibitor. J Thromb Haemost. 2005;3:514–21. - PubMed
-
- Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78:412–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


