Supramolecular architecture of severe acute respiratory syndrome coronavirus revealed by electron cryomicroscopy
- PMID: 16873249
- PMCID: PMC1563832
- DOI: 10.1128/JVI.00645-06
Supramolecular architecture of severe acute respiratory syndrome coronavirus revealed by electron cryomicroscopy
Abstract
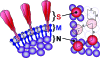
Coronavirus particles are enveloped and pleomorphic and are thus refractory to crystallization and symmetry-assisted reconstruction. A novel methodology of single-particle image analysis was applied to selected virus features to obtain a detailed model of the oligomeric state and spatial relationships among viral structural proteins. Two-dimensional images of the S, M, and N structural proteins of severe acute respiratory syndrome coronavirus and two other coronaviruses were refined to a resolution of approximately 4 nm. Proteins near the viral membrane were arranged in overlapping lattices surrounding a disordered core. Trimeric glycoprotein spikes were in register with four underlying ribonucleoprotein densities. However, the spikes were dispensable for ribonucleoprotein lattice formation. The ribonucleoprotein particles displayed coiled shapes when released from the viral membrane. Our results contribute to the understanding of the assembly pathway used by coronaviruses and other pleomorphic viruses and provide the first detailed view of coronavirus ultrastructure.
Figures






Similar articles
-
Ultrastructure of SARS-CoV, FIPV, and MHV revealed by electron cryomicroscopy.Adv Exp Med Biol. 2006;581:181-5. doi: 10.1007/978-0-387-33012-9_31. Adv Exp Med Biol. 2006. PMID: 17037527 Free PMC article. No abstract available.
-
Probing the structure of the SARS coronavirus using scanning electron microscopy.Antivir Ther. 2004 Apr;9(2):287-9. Antivir Ther. 2004. PMID: 15134191
-
Surface ultrastructure of SARS coronavirus revealed by atomic force microscopy.Cell Microbiol. 2005 Dec;7(12):1763-70. doi: 10.1111/j.1462-5822.2005.00593.x. Cell Microbiol. 2005. PMID: 16309462 Free PMC article.
-
Coronavirus Spike Protein and Tropism Changes.Adv Virus Res. 2016;96:29-57. doi: 10.1016/bs.aivir.2016.08.004. Epub 2016 Sep 13. Adv Virus Res. 2016. PMID: 27712627 Free PMC article. Review.
-
Electron Cryomicroscopy of Viruses at Near-Atomic Resolutions.Annu Rev Virol. 2017 Sep 29;4(1):287-308. doi: 10.1146/annurev-virology-101416-041921. Epub 2017 Jul 17. Annu Rev Virol. 2017. PMID: 28715974 Review.
Cited by
-
Engineering a "muco-trapping" ACE2-immunoglobulin hybrid with picomolar affinity as an inhaled, pan-variant immunotherapy for COVID-19.Bioeng Transl Med. 2024 Feb 7;9(4):e10650. doi: 10.1002/btm2.10650. eCollection 2024 Jul. Bioeng Transl Med. 2024. PMID: 39036085 Free PMC article.
-
Reconstruction of the real 3D shape of the SARS-CoV-2 virus.Biophys J. 2024 May 21;123(10):1297-1310. doi: 10.1016/j.bpj.2024.04.019. Epub 2024 May 6. Biophys J. 2024. PMID: 38715359
-
Emerging Trends of Gold Nanostructures for Point-of-Care Biosensor-Based Detection of COVID-19.Mol Biotechnol. 2024 May 4. doi: 10.1007/s12033-024-01157-y. Online ahead of print. Mol Biotechnol. 2024. PMID: 38703305 Review.
-
Recent SARS-CoV-2 Outlook and Implications in a COVID-19 Vaccination Era.Infect Microbes Dis. 2021 Aug 13;3(3):125-133. doi: 10.1097/IM9.0000000000000072. eCollection 2021 Sep. Infect Microbes Dis. 2021. PMID: 38630122 Free PMC article. Review.
-
The Role of Furin in the Pathogenesis of COVID-19-Associated Neurological Disorders.Life (Basel). 2024 Feb 19;14(2):279. doi: 10.3390/life14020279. Life (Basel). 2024. PMID: 38398788 Free PMC article.
References
-
- Bächi, T. 1980. Intramembrane structural differentiation in Sendai virus maturation. Virology 106:41-49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007354/AI/NIAID NIH HHS/United States
- AI-25913/AI/NIAID NIH HHS/United States
- P41 RR017573/RR/NCRR NIH HHS/United States
- AI-43103/AI/NIAID NIH HHS/United States
- RR17573/RR/NCRR NIH HHS/United States
- R01 AI025913/AI/NIAID NIH HHS/United States
- 266200400058C/PHS HHS/United States
- AI-065359/AI/NIAID NIH HHS/United States
- NS-41219/NS/NINDS NIH HHS/United States
- AI-07354/AI/NIAID NIH HHS/United States
- U54 AI065359/AI/NIAID NIH HHS/United States
- R01 GM066087/GM/NIGMS NIH HHS/United States
- T32 NS041219/NS/NINDS NIH HHS/United States
- GM-066087/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources


