Catalytic and mechanical cycles in F-ATP synthases. Fourth in the Cycles Review Series
- PMID: 16607397
- PMCID: PMC1456893
- DOI: 10.1038/sj.embor.7400646
Catalytic and mechanical cycles in F-ATP synthases. Fourth in the Cycles Review Series
Abstract
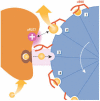
Cycles have a profound role in cellular life at all levels of organization. Well-known cycles in cell metabolism include the tricarboxylic acid and the urea cycle, in which a specific carrier substrate undergoes a sequence of chemical transformations and is regenerated at the end. Other examples include the interconversions of cofactors, such as NADH or ATP, which are present in the cell in limiting amounts and have to be recycled effectively for metabolism to continue. Every living cell performs a rapid turnover of ATP to ADP to fulfil various energetic demands and effectively regenerates the ATP from ADP in an energy-consuming process. The turnover of the ATP cycle is impressive; a human uses about its body weight in ATP per day. Enzymes perform catalytic reaction cycles in which they undergo several chemical and physical transformations before they are converted back to their original states. The ubiquitous F1F(o) ATP synthase is of particular interest not only because of its biological importance, but also owing to its unique rotational mechanism. Here, we give an overview of the membrane-embedded F(o) sector, particularly with respect to the recent crystal structure of the c ring from Ilyobacter tartaricus, and summarize current hypotheses for the mechanism by which rotation of the c ring is generated.
Figures



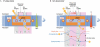



Similar articles
-
Operation mechanism of F(o) F(1)-adenosine triphosphate synthase revealed by its structure and dynamics.IUBMB Life. 2013 Mar;65(3):238-46. doi: 10.1002/iub.1120. Epub 2013 Jan 22. IUBMB Life. 2013. PMID: 23341301 Review.
-
F(1)-ATPase: a prototypical rotary molecular motor.Adv Exp Med Biol. 2012;726:5-16. doi: 10.1007/978-1-4614-0980-9_2. Adv Exp Med Biol. 2012. PMID: 22297508 Review.
-
Coupling H+ transport and ATP synthesis in F1F0-ATP synthases: glimpses of interacting parts in a dynamic molecular machine.J Exp Biol. 1997 Jan;200(Pt 2):217-24. doi: 10.1242/jeb.200.2.217. J Exp Biol. 1997. PMID: 9050229 Review.
-
Structure of the rotor ring of F-Type Na+-ATPase from Ilyobacter tartaricus.Science. 2005 Apr 29;308(5722):659-62. doi: 10.1126/science.1111199. Science. 2005. PMID: 15860619
-
Two distinct proton binding sites in the ATP synthase family.Biochemistry. 2007 Oct 23;46(42):11800-9. doi: 10.1021/bi701083v. Epub 2007 Oct 2. Biochemistry. 2007. PMID: 17910472
Cited by
-
Prevalence of Hypertension and Obesity: Profile of Mitochondrial Function and Markers of Inflammation and Oxidative Stress.Antioxidants (Basel). 2023 Jan 10;12(1):165. doi: 10.3390/antiox12010165. Antioxidants (Basel). 2023. PMID: 36671026 Free PMC article.
-
Measurement of the low-frequency charge noise of bacteria.Phys Rev E. 2022 Jun;105(6-1):064413. doi: 10.1103/PhysRevE.105.064413. Phys Rev E. 2022. PMID: 35854507 Free PMC article.
-
Determinants of Directionality and Efficiency of the ATP Synthase Fo Motor at Atomic Resolution.J Phys Chem Lett. 2022 Jan 13;13(1):387-392. doi: 10.1021/acs.jpclett.1c03358. Epub 2022 Jan 5. J Phys Chem Lett. 2022. PMID: 34985899 Free PMC article.
-
ATP synthase: Evolution, energetics, and membrane interactions.J Gen Physiol. 2020 Nov 2;152(11):e201912475. doi: 10.1085/jgp.201912475. J Gen Physiol. 2020. PMID: 32966553 Free PMC article. Review.
-
Revisiting the protomotive vectorial motion of F0-ATPase.Proc Natl Acad Sci U S A. 2019 Sep 24;116(39):19484-19489. doi: 10.1073/pnas.1909032116. Epub 2019 Sep 11. Proc Natl Acad Sci U S A. 2019. PMID: 31511421 Free PMC article.
References
-
- Abrahams JP, Leslie AG, Lutter R, Walker JE (1994) Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370: 621–628 - PubMed
-
- Boyer PD (1993) The binding change mechanism for ATP synthase—some probabilities and possibilities. Biochim Biophys Acta 1140: 215–250 - PubMed
-
- Boyer PD (1997) The ATP synthase—a splendid molecular machine. Annu Rev Biochem 66: 717–749 - PubMed
-
- Capaldi RA, Aggeler R (2002) Mechanism of the F1Fo-type ATP synthase, a biological rotary motor. Trends Biochem Sci 27: 154–160 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources


