Magnetic resonance spectroscopy in the monitoring of multiple sclerosis
- PMID: 16385018
- PMCID: PMC1351238
- DOI: 10.1177/1051228405284200
Magnetic resonance spectroscopy in the monitoring of multiple sclerosis
Abstract
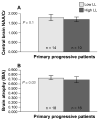
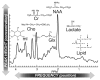
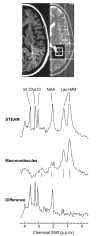
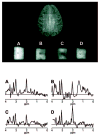
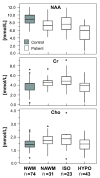
In addition to providing information on tissue structure, magnetic resonance (MR) technology offers the potential to investigate tissue metabolism and function. MR spectroscopy (MRS) offers a wealth of data on the biochemistry of a selected brain tissue volume, which represent potential surrogate markers for the pathology underlying multiple sclerosis (MS). In particular, the N-acetylaspartate peak in an MR spectrum is a putative marker of neuronal and axonal integrity, and the choline peak appears to reflect cell-membrane metabolism. On this basis, a diminished N-acetylaspartate peak is interpreted to represent neuronal/axonal dysfunction or loss, and an elevated choline peak represents heightened cell-membrane turnover, as seen in demyelination, remyelination, inflammation, or gliosis. Therefore, MRS may provide a unique tool to evaluate the severity of MS, establish a prognosis, follow disease evolution, understand its pathogenesis, and evaluate the efficacy of therapeutic interventions, which complements the information obtained from the various forms of assessment made by conventional MR imaging.
Figures

Similar articles
-
Magnetic resonance spectroscopy as a measure of brain damage in multiple sclerosis.J Neurol Sci. 2005 Jun 15;233(1-2):203-8. doi: 10.1016/j.jns.2005.03.018. Epub 2005 Apr 20. J Neurol Sci. 2005. PMID: 15949506 Review.
-
Imaging axonal damage in multiple sclerosis by means of MR spectroscopy.Neurol Sci. 2000;21(4 Suppl 2):S883-7. doi: 10.1007/s100720070031. Neurol Sci. 2000. PMID: 11205368 Review.
-
MR spectroscopy in multiple sclerosis.J Neuroimaging. 2007 Apr;17 Suppl 1:31S-35S. doi: 10.1111/j.1552-6569.2007.00134.x. J Neuroimaging. 2007. PMID: 17425732 Review.
-
In-vivo evidence for stable neuroaxonal damage in the brain of patients with benign multiple sclerosis.Mult Scler. 2009 Jul;15(7):789-94. doi: 10.1177/1352458509103714. Epub 2009 May 22. Mult Scler. 2009. PMID: 19465450
-
Pathogenesis of brain and spinal cord atrophy in multiple sclerosis.J Neuroimaging. 2004 Jul;14(3 Suppl):5S-10S. doi: 10.1177/1051228404266263. J Neuroimaging. 2004. PMID: 15228754 Review.
Cited by
-
T1 mapping from routine 3D T1-weighted inversion recovery sequences in clinical practice: comparison against reference inversion recovery fast field echo T1 scans and feasibility in multiple sclerosis.Neuroradiology. 2024 Oct;66(10):1709-1719. doi: 10.1007/s00234-024-03400-4. Epub 2024 Jun 17. Neuroradiology. 2024. PMID: 38880824
-
Navigating Neural Landscapes: A Comprehensive Review of Magnetic Resonance Imaging (MRI) and Magnetic Resonance Spectroscopy (MRS) Applications in Epilepsy.Cureus. 2024 Mar 25;16(3):e56927. doi: 10.7759/cureus.56927. eCollection 2024 Mar. Cureus. 2024. PMID: 38665706 Free PMC article. Review.
-
Multi-modal analysis of inflammation as a potential mediator of depressive symptoms in young people with HIV: The GOLD depression study.PLoS One. 2024 Feb 22;19(2):e0298787. doi: 10.1371/journal.pone.0298787. eCollection 2024. PLoS One. 2024. PMID: 38386679 Free PMC article.
-
Central nervous system involvement in chronic inflammatory demyelinating polyradiculoneuropathy-MRS and DTI study.Front Neurol. 2024 Jan 25;15:1301405. doi: 10.3389/fneur.2024.1301405. eCollection 2024. Front Neurol. 2024. PMID: 38333607 Free PMC article.
-
The Heterogeneous Multiple Sclerosis Lesion: How Can We Assess and Modify a Degenerating Lesion?Int J Mol Sci. 2023 Jul 5;24(13):11112. doi: 10.3390/ijms241311112. Int J Mol Sci. 2023. PMID: 37446290 Free PMC article. Review.
References
-
- Wolinsky JS, Narayana PA. Magnetic resonance spectroscopy in multiple sclerosis: window into the diseased brain. Curr Opin Neurol. 2002;15:247–251. - PubMed
-
- De Stefano N, Bartolozzi ML, Guidi L, Stromillo ML, Federico A. Magnetic resonance spectroscopy as a measure of brain damage in multiple sclerosis. J Neurol Sci. 2005;233:203–208. - PubMed
-
- Wolinsky JS, Narayana PA, Fenstermacher MJ. Proton magnetic resonance spectroscopy in multiple sclerosis. Neurology. 1990;40:1764–1769. - PubMed
-
- Arnold DL, Matthews PM, Francis G, Antel J. Proton magnetic resonance spectroscopy of human brain in vivo in the evaluation of multiple sclerosis: assessment of the load of disease. Magn Reson Med. 1990;14:154–159. - PubMed
-
- Filippi M, Paty DW, Kappos L, et al. Correlations between changes in disability and T2-weighted brain MRI activity in multiple sclerosis: a follow-up study. Neurology. 1995;45:255–260. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical


