Mice lacking ghrelin receptors resist the development of diet-induced obesity
- PMID: 16322794
- PMCID: PMC1297251
- DOI: 10.1172/JCI26002
Mice lacking ghrelin receptors resist the development of diet-induced obesity
Abstract
Ghrelin is the endogenous ligand for the growth hormone secretagogue receptor (GHSR; ghrelin receptor). Since its discovery, accumulating evidence has suggested that ghrelin may play a role in signaling and reversing states of energy insufficiency. For example, ghrelin levels rise following food deprivation, and ghrelin administration stimulates feeding and increases body weight and adiposity. However, recent loss-of-function studies have raised questions regarding the physiological significance of ghrelin in regulating these processes. Here, we present results of a study using a novel GHSR-null mouse model, in which ghrelin administration fails to acutely stimulate food intake or activate arcuate nucleus neurons. We show that when fed a high-fat diet, both female and male GHSR-null mice eat less food, store less of their consumed calories, preferentially utilize fat as an energy substrate, and accumulate less body weight and adiposity than control mice. Similar effects on body weight and adiposity were also observed in female, but not male, GHSR-null mice fed standard chow. GHSR deletion also affected locomotor activity and levels of glycemia. These findings support the hypothesis that ghrelin-responsive pathways are an important component of coordinated body weight control. Moreover, our data suggest that ghrelin signaling is required for development of the full phenotype of diet-induced obesity.
Figures

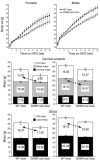
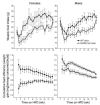
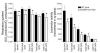
Comment in
-
Is ghrelin a signal for the development of metabolic systems?J Clin Invest. 2005 Dec;115(12):3393-7. doi: 10.1172/JCI27211. J Clin Invest. 2005. PMID: 16322785 Free PMC article.
Similar articles
-
Is ghrelin a signal for the development of metabolic systems?J Clin Invest. 2005 Dec;115(12):3393-7. doi: 10.1172/JCI27211. J Clin Invest. 2005. PMID: 16322785 Free PMC article.
-
Absence of ghrelin protects against early-onset obesity.J Clin Invest. 2005 Dec;115(12):3573-8. doi: 10.1172/JCI26003. J Clin Invest. 2005. PMID: 16322795 Free PMC article.
-
Rats with a truncated ghrelin receptor (GHSR) do not respond to ghrelin, and show reduced intake of palatable, high-calorie food.Physiol Behav. 2016 Sep 1;163:88-96. doi: 10.1016/j.physbeh.2016.04.048. Epub 2016 Apr 27. Physiol Behav. 2016. PMID: 27129673
-
Ghrelin and the short- and long-term regulation of appetite and body weight.Physiol Behav. 2006 Aug 30;89(1):71-84. doi: 10.1016/j.physbeh.2006.05.022. Epub 2006 Jul 21. Physiol Behav. 2006. PMID: 16859720 Review.
-
Brain somatic cross-talk: ghrelin, leptin and ultimate challengers of obesity.Nutr Neurosci. 2005 Feb;8(1):1-5. doi: 10.1080/10284150400027107. Nutr Neurosci. 2005. PMID: 15909762 Review.
Cited by
-
The LEAP2 Response to Cancer-Related Anorexia-Cachexia Syndrome in Male Mice and Patients.Endocrinology. 2024 Sep 26;165(11):bqae132. doi: 10.1210/endocr/bqae132. Endocrinology. 2024. PMID: 39331742
-
Hunger signalling in the olfactory bulb primes exploration, food-seeking and peripheral metabolism.Mol Metab. 2024 Nov;89:102025. doi: 10.1016/j.molmet.2024.102025. Epub 2024 Sep 3. Mol Metab. 2024. PMID: 39236785 Free PMC article.
-
Leptin and ghrelin dynamics: unraveling their influence on food intake, energy balance, and the pathophysiology of type 2 diabetes mellitus.J Diabetes Metab Disord. 2024 Apr 18;23(1):427-440. doi: 10.1007/s40200-024-01418-2. eCollection 2024 Jun. J Diabetes Metab Disord. 2024. PMID: 38932792 Review.
-
Genetic or pharmacological GHSR blockade has sexually dimorphic effects in rodents on a high-fat diet.Commun Biol. 2024 May 25;7(1):632. doi: 10.1038/s42003-024-06303-5. Commun Biol. 2024. PMID: 38796563 Free PMC article.
-
Role of ghrelin hormone in the development of alcohol-associated liver disease.Biomed Pharmacother. 2024 May;174:116595. doi: 10.1016/j.biopha.2024.116595. Epub 2024 Apr 19. Biomed Pharmacother. 2024. PMID: 38640709
References
-
- Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. - PubMed
-
- Cummings DE, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. - PubMed
-
- Cummings DE, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med. 2002;346:1623–1630. - PubMed
-
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. - PubMed
-
- Nakazato M, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1F32DK64564-01/DK/NIDDK NIH HHS/United States
- 5T32DK07516/DK/NIDDK NIH HHS/United States
- P30DK57521/DK/NIDDK NIH HHS/United States
- R37 DK053301/DK/NIDDK NIH HHS/United States
- DK59630/DK/NIDDK NIH HHS/United States
- DK56116/DK/NIDDK NIH HHS/United States
- F32 DK064564/DK/NIDDK NIH HHS/United States
- R01 DK053301/DK/NIDDK NIH HHS/United States
- P01 DK056116/DK/NIDDK NIH HHS/United States
- DK53301/DK/NIDDK NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- U24 DK059630/DK/NIDDK NIH HHS/United States
- T32 DK007516/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases


