Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability
- PMID: 16001073
- PMCID: PMC2784913
- DOI: 10.1038/nature03688
Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability
Abstract
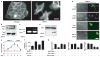
The tumour microenvironment can be a potent carcinogen, not only by facilitating cancer progression and activating dormant cancer cells, but also by stimulating tumour formation. We have previously investigated stromelysin-1/matrix metalloproteinase-3 (MMP-3), a stromal enzyme upregulated in many breast tumours, and found that MMP-3 can cause epithelial-mesenchymal transition (EMT) and malignant transformation in cultured cells, and genomically unstable mammary carcinomas in transgenic mice. Here we explain the molecular pathways by which MMP-3 exerts these effects: exposure of mouse mammary epithelial cells to MMP-3 induces the expression of an alternatively spliced form of Rac1, which causes an increase in cellular reactive oxygen species (ROS). The ROS stimulate the expression of the transcription factor Snail and EMT, and cause oxidative damage to DNA and genomic instability. These findings identify a previously undescribed pathway in which a component of the breast tumour microenvironment alters cellular structure in culture and tissue structure in vivo, leading to malignant transformation.
Figures


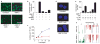
Similar articles
-
Matrix metalloproteinase-induced malignancy in mammary epithelial cells.Cells Tissues Organs. 2007;185(1-3):104-10. doi: 10.1159/000101310. Cells Tissues Organs. 2007. PMID: 17587815 Review.
-
ROS-induced epithelial-mesenchymal transition in mammary epithelial cells is mediated by NF-kB-dependent activation of Snail.Oncotarget. 2014 May 15;5(9):2827-38. doi: 10.18632/oncotarget.1940. Oncotarget. 2014. PMID: 24811539 Free PMC article.
-
Matrix metalloproteinase induction of Rac1b, a key effector of lung cancer progression.Sci Transl Med. 2012 Jul 11;4(142):142ra95. doi: 10.1126/scitranslmed.3004062. Sci Transl Med. 2012. PMID: 22786680 Free PMC article.
-
Involvement of hnRNP A1 in the matrix metalloprotease-3-dependent regulation of Rac1 pre-mRNA splicing.J Cell Biochem. 2012 Jul;113(7):2319-29. doi: 10.1002/jcb.24103. J Cell Biochem. 2012. PMID: 22345078 Free PMC article.
-
Matrix metalloproteinase-induced epithelial-mesenchymal transition: tumor progression at Snail's pace.Int J Biochem Cell Biol. 2007;39(6):1082-8. doi: 10.1016/j.biocel.2007.03.002. Epub 2007 Mar 12. Int J Biochem Cell Biol. 2007. PMID: 17416542 Review.
Cited by
-
BAIAP2L2 promotes the malignancy of hepatocellular carcinoma via GABPB1-mediated reactive oxygen species imbalance.Cancer Gene Ther. 2024 Dec;31(12):1868-1883. doi: 10.1038/s41417-024-00841-0. Epub 2024 Nov 4. Cancer Gene Ther. 2024. PMID: 39496939
-
Harnessing quantum light for microscopic biomechanical imaging of cells and tissues.Proc Natl Acad Sci U S A. 2024 Nov 5;121(45):e2413938121. doi: 10.1073/pnas.2413938121. Epub 2024 Oct 31. Proc Natl Acad Sci U S A. 2024. PMID: 39480851 Free PMC article.
-
The Role and Function of Secretory Protein Matrix Metalloproteinase-3 (MMP3) in Cervical Cancer.Iran J Public Health. 2024 Apr;53(4):855-866. doi: 10.18502/ijph.v53i4.15562. Iran J Public Health. 2024. PMID: 39444485 Free PMC article.
-
Steering research on mRNA splicing in cancer towards clinical translation.Nat Rev Cancer. 2024 Dec;24(12):887-905. doi: 10.1038/s41568-024-00750-2. Epub 2024 Oct 9. Nat Rev Cancer. 2024. PMID: 39384951 Review.
-
Defining precancer: a grand challenge for the cancer community.Nat Rev Cancer. 2024 Nov;24(11):792-809. doi: 10.1038/s41568-024-00744-0. Epub 2024 Oct 1. Nat Rev Cancer. 2024. PMID: 39354069 Review.
References
-
- Lochter A, et al. Misregulation of stromelysin-1 expression in mouse mammary tumour cells accompanies acquisition of stromelysin-1-dependent invasive properties. J Biol Chem. 1997;272:5007–5015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

