Development and characterization of a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody that provides effective immunoprophylaxis in mice
- PMID: 15655773
- PMCID: PMC7110081
- DOI: 10.1086/427242
Development and characterization of a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody that provides effective immunoprophylaxis in mice
Abstract
Background: Severe acute respiratory syndrome (SARS) remains a significant public health concern after the epidemic in 2003. Human monoclonal antibodies (MAbs) that neutralize SARS-associated coronavirus (SARS-CoV) could provide protection for exposed individuals.
Methods: Transgenic mice with human immunoglobulin genes were immunized with the recombinant major surface (S) glycoprotein ectodomain of SARS-CoV. Epitopes of 2 neutralizing MAbs derived from these mice were mapped and evaluated in a murine model of SARS-CoV infection.
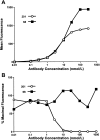
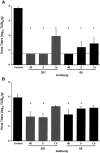
Results: Both MAbs bound to S glycoprotein expressed on transfected cells but differed in their ability to block binding of S glycoprotein to Vero E6 cells. Immunoprecipitation analysis revealed 2 antibody-binding epitopes: one MAb (201) bound within the receptor-binding domain at aa 490-510, and the other MAb (68) bound externally to the domain at aa 130-150. Mice that received 40 mg/kg of either MAb prior to challenge with SARS-CoV were completely protected from virus replication in the lungs, and doses as low as 1.6 mg/kg offered significant protection.
Conclusions: Two neutralizing epitopes were defined for MAbs to SARS-CoV S glycoprotein. Antibodies to both epitopes protected mice against SARS-CoV challenge. Clinical trials are planned to test MAb 201, a fully human MAb specific for the epitope within the receptor-binding region.
Figures


Similar articles
-
Structural basis for potent cross-neutralizing human monoclonal antibody protection against lethal human and zoonotic severe acute respiratory syndrome coronavirus challenge.J Virol. 2008 Apr;82(7):3220-35. doi: 10.1128/JVI.02377-07. Epub 2008 Jan 16. J Virol. 2008. PMID: 18199635 Free PMC article.
-
Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants.PLoS Med. 2006 Jul;3(7):e237. doi: 10.1371/journal.pmed.0030237. PLoS Med. 2006. PMID: 16796401 Free PMC article.
-
Molecular and biological characterization of human monoclonal antibodies binding to the spike and nucleocapsid proteins of severe acute respiratory syndrome coronavirus.J Virol. 2005 Feb;79(3):1635-44. doi: 10.1128/JVI.79.3.1635-1644.2005. J Virol. 2005. PMID: 15650189 Free PMC article.
-
Structural Analysis of Neutralizing Epitopes of the SARS-CoV-2 Spike to Guide Therapy and Vaccine Design Strategies.Viruses. 2021 Jan 19;13(1):134. doi: 10.3390/v13010134. Viruses. 2021. PMID: 33477902 Free PMC article. Review.
-
Neutralizing human monoclonal antibodies to severe acute respiratory syndrome coronavirus: target, mechanism of action, and therapeutic potential.Rev Med Virol. 2012 Jan;22(1):2-17. doi: 10.1002/rmv.706. Epub 2011 Sep 8. Rev Med Virol. 2012. PMID: 21905149 Free PMC article. Review.
Cited by
-
Antiviral Protein-Protein Interaction Inhibitors.J Med Chem. 2024 Mar 14;67(5):3205-3231. doi: 10.1021/acs.jmedchem.3c01543. Epub 2024 Feb 23. J Med Chem. 2024. PMID: 38394369 Free PMC article. Review.
-
COVID-19 vaccine acceptance and its drivers among Pakistani population.Pak J Med Sci. 2023 Mar-Apr;39(2):553-556. doi: 10.12669/pjms.39.2.6051. Pak J Med Sci. 2023. PMID: 36950442 Free PMC article.
-
Anti-SARS-CoV-2 immunoadhesin remains effective against Omicron and other emerging variants of concern.iScience. 2022 Oct 21;25(10):105193. doi: 10.1016/j.isci.2022.105193. Epub 2022 Sep 28. iScience. 2022. PMID: 36188189 Free PMC article.
-
Immune Response to SARS-CoV-2 Vaccines.Biomedicines. 2022 Jun 21;10(7):1464. doi: 10.3390/biomedicines10071464. Biomedicines. 2022. PMID: 35884770 Free PMC article. Review.
-
Animal models for studying coronavirus infections and developing antiviral agents and vaccines.Antiviral Res. 2022 Jul;203:105345. doi: 10.1016/j.antiviral.2022.105345. Epub 2022 May 21. Antiviral Res. 2022. PMID: 35605699 Free PMC article. Review.
References
-
- Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76. - PubMed
-
- Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. - PubMed
-
- World Health Organization (WHO) China confirms SARS infection in another previously reported case; summary of cases to date—update 5 2004. Geneva: WHO; [Accessed 3 January 2005]. http://www.who.int/csr/don/2004_04_30/en.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous


