CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate
- PMID: 15381732
- PMCID: PMC2211970
- DOI: 10.1084/jem.20041069
CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate
Erratum in
- J Exp Med. 2004 Oct 18;200(8):following 1089
Abstract
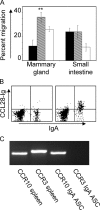
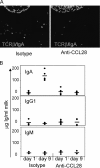
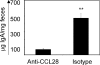
The accumulation of immunoglobulin (Ig)A antibody-secreting cells (ASCs) in the lactating mammary gland leads to secretion of antibodies into milk and their passive transfer to the suckling newborn. This transfer of IgA from mother to infant provides transient immune protection against a variety of gastrointestinal pathogens. Here we show that the mucosal epithelial chemokine CCL28 is up-regulated in the mammary gland during lactation and that IgA ASCs from this tissue express CCR10 and migrate to CCL28. In vivo treatment with anti-CCL28 antibody blocks IgA ASC accumulation in the mammary gland, inhibiting IgA antibody secretion into milk and the subsequent appearance of antibody in the gastrointestinal tract of nursing neonates. We propose that CCL28 is a key regulator of IgA ASC accumulation in the mammary gland and thus controls the passive transfer of IgA antibodies from mother to infant.
Figures

Similar articles
-
An increase in milk IgA correlates with both pIgR expression and IgA plasma cell accumulation in the lactating mammary gland of PRM/Alf mice.J Reprod Immunol. 2012 Dec;96(1-2):25-33. doi: 10.1016/j.jri.2012.08.001. Epub 2012 Sep 25. J Reprod Immunol. 2012. PMID: 23021255
-
Supplemental β-carotene increases IgA-secreting cells in mammary gland and IgA transfer from milk to neonatal mice.Br J Nutr. 2011 Jan;105(1):24-30. doi: 10.1017/S0007114510003089. Epub 2010 Aug 23. Br J Nutr. 2011. PMID: 20727240
-
Molecular cloning and functional characterization of porcine CCL28: possible involvement in homing of IgA antibody secreting cells into the mammary gland.Mol Immunol. 2008 Jan;45(1):271-7. doi: 10.1016/j.molimm.2007.04.026. Epub 2007 Jun 11. Mol Immunol. 2008. PMID: 17561257
-
Immunological functions of the mammary gland and its secretion--comparative review.Aust J Biol Sci. 1980 Aug;33(4):403-22. doi: 10.1071/bi9800403. Aust J Biol Sci. 1980. PMID: 7004419 Review.
-
Mucosal immunity of the mammary gland and immunology of mother/newborn interrelation.Arch Immunol Ther Exp (Warsz). 1990;38(1-2):145-64. Arch Immunol Ther Exp (Warsz). 1990. PMID: 2288471 Review.
Cited by
-
Inter-individual and inter-regional variability of breast milk antibody reactivity to bacterial lipopolysaccharides.Front Immunol. 2024 Sep 6;15:1404192. doi: 10.3389/fimmu.2024.1404192. eCollection 2024. Front Immunol. 2024. PMID: 39308863 Free PMC article.
-
The right educational environment: Oral tolerance in early life.Immunol Rev. 2024 Sep;326(1):17-34. doi: 10.1111/imr.13366. Epub 2024 Jul 13. Immunol Rev. 2024. PMID: 39001685 Review.
-
Protection from environmental enteric dysfunction and growth improvement in malnourished newborns by amplification of secretory IgA.Cell Rep Med. 2024 Jul 16;5(7):101639. doi: 10.1016/j.xcrm.2024.101639. Epub 2024 Jul 2. Cell Rep Med. 2024. PMID: 38959887 Free PMC article.
-
Early life imprinting of intestinal immune tolerance and tissue homeostasis.Immunol Rev. 2024 May;323(1):303-315. doi: 10.1111/imr.13321. Epub 2024 Mar 19. Immunol Rev. 2024. PMID: 38501766 Review.
-
Stability and heterogeneity in the antimicrobiota reactivity of human milk-derived immunoglobulin A.J Exp Med. 2023 Aug 7;220(8):e20220839. doi: 10.1084/jem.20220839. Epub 2023 Jul 18. J Exp Med. 2023. PMID: 37462916 Free PMC article.
References
-
- Williams, R.C., and R.J. Gibbons. 1972. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 177:697–699. - PubMed
-
- Russell, M.W., M. Kilian, and M.E. Lamm. 1999. Biological activities of IgA. Mucosal Immunology. P.L. Ogra, J. Mestecky, M.E. Lamm, W. Strober, J. Bienenstock, and J.R. McGhee, editors. Academic Press, San Diego. 225–240.
-
- Holmgren, J., L.A. Hanson, B. Carlson, B.S. Lindblad, and J. Rahimtoola. 1976. Neutralizing antibodies against Escherichia coli and Vibrio cholerae enterotoxins in human milk from a developing country. Scand. J. Immunol. 5:867–871. - PubMed
-
- Stoliar, O.A., R.P. Pelley, E. Kaniecki-Green, M.H. Kkaus, and C.C. Carpenter. 1976. Secretory IgA against enterotoxins in breast-milk. Lancet. 1:1258–1261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous


