A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine
- PMID: 15317861
- PMCID: PMC6729781
- DOI: 10.1523/JNEUROSCI.1850-04.2004
A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine
Abstract
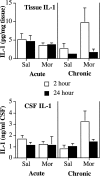
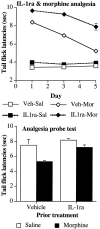
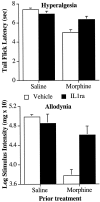
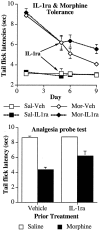
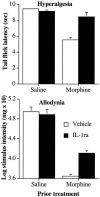
The present experiments examined the role of spinal proinflammatory cytokines [interleukin-1beta (IL-1)] and chemokines (fractalkine) in acute analgesia and in the development of analgesic tolerance, thermal hyperalgesia, and tactile allodynia in response to chronic intrathecal morphine. Chronic (5 d), but not acute (1 d), intrathecal morphine was associated with a rapid increase in proinflammatory cytokine protein and/or mRNA in dorsal spinal cord and lumbosacral CSF. To determine whether IL-1 release modulates the effects of morphine, intrathecal morphine was coadministered with intrathecal IL-1 receptor antagonist (IL-1ra). This regimen potentiated acute morphine analgesia and inhibited the development of hyperalgesia, allodynia, and analgesic tolerance. Similarly, intrathecal IL-1ra administered after the establishment of morphine tolerance reversed hyperalgesia and prevented the additional development of tolerance and allodynia. Fractalkine also appears to modulate the effects of intrathecal morphine because coadministration of morphine with intrathecal neutralizing antibody against the fractalkine receptor (CX3CR1) potentiated acute morphine analgesia and attenuated the development of tolerance, hyperalgesia, and allodynia. Fractalkine may be exerting these effects via IL-1 because fractalkine (CX3CL1) induced the release of IL-1 from acutely isolated dorsal spinal cord in vitro. Finally, gene therapy with an adenoviral vector encoding for the release of the anti-inflammatory cytokine IL-10 also potentiated acute morphine analgesia and attenuated the development of tolerance, hyperalgesia, and allodynia. Taken together, these results suggest that IL-1 and fractalkine are endogenous regulators of morphine analgesia and are involved in the increases in pain sensitivity that occur after chronic opiates.
Figures


Similar articles
-
Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia.Brain Behav Immun. 2008 Nov;22(8):1178-89. doi: 10.1016/j.bbi.2008.05.004. Epub 2008 Jul 2. Brain Behav Immun. 2008. PMID: 18599265 Free PMC article.
-
An initial investigation of spinal mechanisms underlying pain enhancement induced by fractalkine, a neuronally released chemokine.Eur J Neurosci. 2005 Dec;22(11):2775-82. doi: 10.1111/j.1460-9568.2005.04470.x. Eur J Neurosci. 2005. PMID: 16324111
-
Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats.Eur J Neurosci. 2004 Nov;20(9):2294-302. doi: 10.1111/j.1460-9568.2004.03709.x. Eur J Neurosci. 2004. PMID: 15525271
-
Implications of intrathecal pertussis toxin animal model on the cellular mechanisms of neuropathic pain syndrome.Acta Anaesthesiol Sin. 2003 Dec;41(4):187-96. Acta Anaesthesiol Sin. 2003. PMID: 14768516 Review.
-
[Fractalkine and inflammatory diseases].Nihon Rinsho Meneki Gakkai Kaishi. 2005 Jun;28(3):131-9. doi: 10.2177/jsci.28.131. Nihon Rinsho Meneki Gakkai Kaishi. 2005. PMID: 15997176 Review. Japanese.
Cited by
-
Psilocybin reduces heroin seeking behavior and modulates inflammatory gene expression in the nucleus accumbens and prefrontal cortex of male rats.Mol Psychiatry. 2024 Oct 21. doi: 10.1038/s41380-024-02788-y. Online ahead of print. Mol Psychiatry. 2024. PMID: 39433903
-
Psilocybin reduces heroin seeking behavior and modulates inflammatory gene expression in the nucleus accumbens and prefrontal cortex of male rats.bioRxiv [Preprint]. 2024 Jun 1:2024.05.28.596205. doi: 10.1101/2024.05.28.596205. bioRxiv. 2024. Update in: Mol Psychiatry. 2024 Oct 21. doi: 10.1038/s41380-024-02788-y. PMID: 38854027 Free PMC article. Updated. Preprint.
-
Promising immunomodulators for management of substance and alcohol use disorders.Expert Opin Pharmacother. 2024 May;25(7):867-884. doi: 10.1080/14656566.2024.2360653. Epub 2024 May 31. Expert Opin Pharmacother. 2024. PMID: 38803314 Review.
-
Morphine-Driven m6A Epitranscriptomic Neuroadaptations in Primary Cortical Cultures.Mol Neurobiol. 2024 Dec;61(12):10684-10704. doi: 10.1007/s12035-024-04219-z. Epub 2024 May 23. Mol Neurobiol. 2024. PMID: 38780720 Free PMC article.
-
CC Chemokine Family Members' Modulation as a Novel Approach for Treating Central Nervous System and Peripheral Nervous System Injury-A Review of Clinical and Experimental Findings.Int J Mol Sci. 2024 Mar 28;25(7):3788. doi: 10.3390/ijms25073788. Int J Mol Sci. 2024. PMID: 38612597 Free PMC article. Review.
References
-
- Apte RN, Durum SK, Oppenheim JJ (1990) Opioids modulate interleukin-1 production and secretion by bone-marrow macrophages. Immunol Lett 24: 141-148. - PubMed
-
- Azuma Y, Ohura K (2002) Endomorphin-2 modulates productions of TNF-alpha, IL-1beta, IL-10, and IL-12, and alters functions related to innate immune of macrophages. Inflammation 26: 223-232. - PubMed
-
- Beitner-Johnson D, Guitart X, Nestler EJ (1993) Glial fibrillary acidic protein and the mesolimbic dopamine system: regulation by chronic morphine and Lewis-Fischer strain differences in the rat ventral tegmental area. J Neurochem 61: 1766-1773. - PubMed
-
- Boddeke EW (2001) Involvement of chemokines in pain. Eur J Pharmacol 429: 115-119. - PubMed
-
- Brewer KL, Bethea JR, Yezierski RP (1999) Neuroprotective effects of interleukin-10 following excitotoxic spinal cord injury. Exp Neurol 159: 484-493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

