Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection
- PMID: 14633608
- PMCID: PMC1892369
- DOI: 10.1016/S0002-9440(10)63591-2
Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection
Abstract
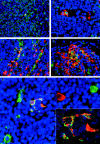
Ebola virus (EBOV) infection causes a severe and fatal hemorrhagic disease that in many ways appears to be similar in humans and nonhuman primates; however, little is known about the development of EBOV hemorrhagic fever. In the present study, 21 cynomolgus monkeys were experimentally infected with EBOV and examined sequentially over a 6-day period to investigate the pathological events of EBOV infection that lead to death. Importantly, dendritic cells in lymphoid tissues were identified as early and sustained targets of EBOV, implicating their important role in the immunosuppression characteristic of EBOV infections. Bystander lymphocyte apoptosis, previously described in end-stage tissues, occurred early in the disease-course in intravascular and extravascular locations. Of note, apoptosis and loss of NK cells was a prominent finding, suggesting the importance of innate immunity in determining the fate of the host. Analysis of peripheral blood mononuclear cell gene expression showed temporal increases in tumor necrosis factor-related apoptosis-inducing ligand and Fas transcripts, revealing a possible mechanism for the observed bystander apoptosis, while up-regulation of NAIP and cIAP2 mRNA suggest that EBOV has evolved additional mechanisms to resist host defenses by inducing protective transcripts in cells that it infects. The sequence of pathogenetic events identified in this study should provide new targets for rational prophylactic and chemotherapeutic interventions.
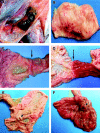
Figures





Similar articles
-
Pathogenesis of Marburg hemorrhagic fever in cynomolgus macaques.J Infect Dis. 2011 Nov;204 Suppl 3:S1021-31. doi: 10.1093/infdis/jir339. J Infect Dis. 2011. PMID: 21987738
-
Proinflammatory response during Ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily.Immunol Lett. 2002 Mar 1;80(3):169-79. doi: 10.1016/s0165-2478(01)00327-3. Immunol Lett. 2002. PMID: 11803049
-
Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells.Am J Pathol. 2003 Dec;163(6):2371-82. doi: 10.1016/S0002-9440(10)63592-4. Am J Pathol. 2003. PMID: 14633609 Free PMC article.
-
Ebola virus: unravelling pathogenesis to combat a deadly disease.Trends Mol Med. 2006 May;12(5):206-15. doi: 10.1016/j.molmed.2006.03.006. Epub 2006 Apr 17. Trends Mol Med. 2006. PMID: 16616875 Review.
-
Ebola virus: new insights into disease aetiopathology and possible therapeutic interventions.Expert Rev Mol Med. 2004 Sep 21;6(20):1-24. doi: 10.1017/S1462399404008300. Expert Rev Mol Med. 2004. PMID: 15383160 Review.
Cited by
-
Emerging and reemerging infectious diseases: global trends and new strategies for their prevention and control.Signal Transduct Target Ther. 2024 Sep 11;9(1):223. doi: 10.1038/s41392-024-01917-x. Signal Transduct Target Ther. 2024. PMID: 39256346 Free PMC article. Review.
-
Vaccinomics: Paving the Way for Personalized Immunization.Curr Pharm Des. 2024;30(13):1031-1047. doi: 10.2174/0113816128280417231204085137. Curr Pharm Des. 2024. PMID: 38898820 Review.
-
Vascular dysfunction in hemorrhagic viral fevers: opportunities for organotypic modeling.Biofabrication. 2024 Jun 5;16(3):032008. doi: 10.1088/1758-5090/ad4c0b. Biofabrication. 2024. PMID: 38749416 Free PMC article. Review.
-
Rhabdomyolysis, Acute Kidney Injury, and Mortality in Ebola Virus Disease: Retrospective Analysis of Cases From the Eastern Democratic Republic of the Congo, 2019.J Infect Dis. 2024 Aug 16;230(2):e465-e473. doi: 10.1093/infdis/jiae224. J Infect Dis. 2024. PMID: 38696335 Free PMC article.
-
Natural history of Ebola virus disease in rhesus monkeys shows viral variant emergence dynamics and tissue-specific host responses.Cell Genom. 2023 Nov 21;3(12):100440. doi: 10.1016/j.xgen.2023.100440. eCollection 2023 Dec 13. Cell Genom. 2023. PMID: 38169842 Free PMC article.
References
-
- Bowen ETW, Lloyd G, Harris WJ, Platt GS, Baskerville A, Vella EE: Viral haemorrhagic fever in southern Sudan and northern Zaire. Lancet 1977, 1:571-573 - PubMed
-
- Johnson KM, Lange JV, Webb PA, Murphy FA: Isolation and partial characterization of a new virus causing acute haemorrhagic fever in Zaire. Lancet 1977, 1:569-571 - PubMed
-
- Khan AS, Tshioko K, Heymann DL, Le Guenno B, Nabreth P, Kerstiens B, Fleerackers Y, Kilmarx PH, Rodier GR, Nkuku O, Rollin PE, Sanchez A, Zaki SR, Swanepoel R, Tomori O, Nichol ST, Peters CJ, Muyembe-Tamfum JJ, Ksiazek TG: The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. J Infect Dis 1999, 179(Suppl 1):S76-S86 - PubMed
-
- Bowen ETW, Platt GS, Simpson DIH, McArdell LB, Raymond RT: Ebola haemorrhagic fever: experimental infection of monkeys. Trans R Soc Trop Med Hyg 1978, 72:188-191 - PubMed
-
- Fisher-Hoch SP, Brammer LT, Trappier SG, Hutwagner LC, Farrar BB, Ruo SL, Brown BG, Hermann LM, Perez-Oronoz GI, Goldsmith CS, Hanes MA, McCormick JB: Pathogenic potential of filoviruses: role of geographic origin of primate host and virus strain. J Infect Dis 1992, 166:753-763 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous


