The rate of hydrolysis of phosphomonoester dianions and the exceptional catalytic proficiencies of protein and inositol phosphatases
- PMID: 12721374
- PMCID: PMC156248
- DOI: 10.1073/pnas.0631607100
The rate of hydrolysis of phosphomonoester dianions and the exceptional catalytic proficiencies of protein and inositol phosphatases
Abstract
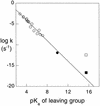
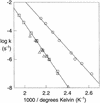
To evaluate the proficiency of phosphatases as catalysts, the rate of the uncatalyzed hydrolysis of simple phosphate monoester dianions was estimated by extrapolating rates measured over a range of high temperatures. The rate of spontaneous hydrolysis of phenyl phosphate dianion indicates that a linear free energy relationship reported earlier is reliable for leaving groups whose conjugate acids have pKa values up to at least 10. Using Teflon reaction vessels, it proved possible to follow the hydrolysis of methyl phosphate and 3-(4-carboxy)-2,2-dimethylpropyl phosphate in strong alkali. Even in 1 M KOH, the reaction was found to be specific acid catalyzed. These results establish an upper limit for dianion reactivity, which had been overestimated earlier as a result of the leaching by alkali of silicic acid from quartz reaction vessels. The present findings indicate that the half-time for attack by water on alkyl phosphate dianions is 1.1 x 10(12) years (k = 2 x 10(-20) s) at 25 degrees C and that phosphatases involved in cell signaling and regulation produce the largest rate enhancements that have been identified thus far. Protein phosphatase-1 and inositol 1-phosphatase exceed all other known enzymes in their affinities for the altered substrates in the transition state.
Figures
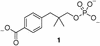

Similar articles
-
Models for biological phosphoryl transfer.Biochim Biophys Acta. 2004 Mar 11;1697(1-2):279-87. doi: 10.1016/j.bbapap.2003.11.031. Biochim Biophys Acta. 2004. PMID: 15023368 Review.
-
Mechanistic studies of protein tyrosine phosphatases YopH and Cdc25A with m-nitrobenzyl phosphate.Biochemistry. 2004 Jun 29;43(25):8256-64. doi: 10.1021/bi0496182. Biochemistry. 2004. PMID: 15209522
-
Theoretical evaluation of the substrate-assisted catalysis mechanism for the hydrolysis of phosphate monoester dianions.Chemistry. 2007;13(13):3617-29. doi: 10.1002/chem.200601458. Chemistry. 2007. PMID: 17290469
-
Calcineurin-catalyzed reaction with phosphite and phosphate esters of tyrosine.Biochemistry. 1991 Mar 26;30(12):3019-24. doi: 10.1021/bi00226a006. Biochemistry. 1991. PMID: 1848782
-
Inhibitors of Serine/Threonine Protein Phosphatases: Biochemical and Structural Studies Provide Insight for Further Development.Curr Med Chem. 2019;26(15):2634-2660. doi: 10.2174/0929867325666180508095242. Curr Med Chem. 2019. PMID: 29737249 Free PMC article. Review.
Cited by
-
General instability of dipeptides in concentrated sulfuric acid as relevant for the Venus cloud habitability.Sci Rep. 2024 Jul 24;14(1):17083. doi: 10.1038/s41598-024-67342-w. Sci Rep. 2024. PMID: 39048621 Free PMC article.
-
Prebiotic chemical reactivity in solution with quantum accuracy and microsecond sampling using neural network potentials.Proc Natl Acad Sci U S A. 2024 Jun 4;121(23):e2322040121. doi: 10.1073/pnas.2322040121. Epub 2024 May 29. Proc Natl Acad Sci U S A. 2024. PMID: 38809704
-
Dietary potassium stimulates Ppp1Ca-Ppp1r1a dephosphorylation of kidney NaCl cotransporter and reduces blood pressure.J Clin Invest. 2023 Nov 1;133(21):e158498. doi: 10.1172/JCI158498. J Clin Invest. 2023. PMID: 37676724 Free PMC article.
-
Decoupling of catalysis and transition state analog binding from mutations throughout a phosphatase revealed by high-throughput enzymology.Proc Natl Acad Sci U S A. 2023 Jul 18;120(29):e2219074120. doi: 10.1073/pnas.2219074120. Epub 2023 Jul 10. Proc Natl Acad Sci U S A. 2023. PMID: 37428919 Free PMC article.
-
Solvent and solvation effects on reactivities and mechanisms of phospho group transfers from phosphate and phosphinate esters to nucleophiles.Front Chem. 2023 Apr 27;11:1176746. doi: 10.3389/fchem.2023.1176746. eCollection 2023. Front Chem. 2023. PMID: 37179775 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


