Saccharomyces cerevisiae DNA polymerase epsilon and polymerase sigma interact physically and functionally, suggesting a role for polymerase epsilon in sister chromatid cohesion
- PMID: 12665575
- PMCID: PMC152548
- DOI: 10.1128/MCB.23.8.2733-2748.2003
Saccharomyces cerevisiae DNA polymerase epsilon and polymerase sigma interact physically and functionally, suggesting a role for polymerase epsilon in sister chromatid cohesion
Abstract
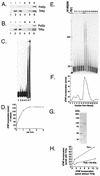
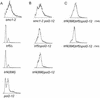
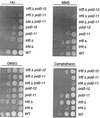
The large subunit of Saccharomyces cerevisiae DNA polymerase epsilon, Pol2, comprises two essential functions. The N terminus has essential DNA polymerase activity. The C terminus is also essential, but its function is unknown. We report here that the C-terminal domain of Pol2 interacts with polymerase sigma (Pol sigma), a recently identified, essential nuclear nucleotidyl transferase encoded by two redundant genes, TRF4 and TRF5. This interaction is functional, since Pol sigma stimulates the polymerase activity of the Pol epsilon holoenzyme significantly. Since Trf4 is required for sister chromatid cohesion as well as for completion of S phase and repair, the interaction suggested that Pol epsilon, like Pol sigma, might form a link between the replication apparatus and sister chromatid cohesion and/or repair machinery. We present evidence that pol2 mutants are defective in sister chromatid cohesion. In addition, Pol2 interacts with SMC1, a subunit of the cohesin complex, and with ECO1/CTF7, required for establishing sister chromatid cohesion; and pol2 mutations act synergistically with smc1 and scc1. We also show that trf5 Delta mutants, like trf4 Delta mutants, are defective in DNA repair and sister chromatid cohesion.
Figures




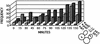
Similar articles
-
Pol kappa: A DNA polymerase required for sister chromatid cohesion.Science. 2000 Aug 4;289(5480):774-9. doi: 10.1126/science.289.5480.774. Science. 2000. PMID: 10926539
-
Chl1p, a DNA helicase-like protein in budding yeast, functions in sister-chromatid cohesion.Genetics. 2004 Jan;166(1):33-42. doi: 10.1534/genetics.166.1.33. Genetics. 2004. PMID: 15020404 Free PMC article.
-
Structure/function analysis of the Saccharomyces cerevisiae Trf4/Pol sigma DNA polymerase.Genetics. 2002 Feb;160(2):381-91. doi: 10.1093/genetics/160.2.381. Genetics. 2002. PMID: 11861546 Free PMC article.
-
Evidence that replication fork components catalyze establishment of cohesion between sister chromatids.Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8270-5. doi: 10.1073/pnas.131022798. Proc Natl Acad Sci U S A. 2001. PMID: 11459963 Free PMC article. Review.
-
Replication-related activities establish cohesion between sister chromatids.Cell Biochem Biophys. 2001;35(3):289-301. doi: 10.1385/CBB:35:3:289. Cell Biochem Biophys. 2001. PMID: 11894848 Review.
Cited by
-
Replisome-cohesin interactions provided by the Tof1-Csm3 and Mrc1 cohesion establishment factors.Chromosoma. 2023 Jun;132(2):117-135. doi: 10.1007/s00412-023-00797-4. Epub 2023 May 11. Chromosoma. 2023. PMID: 37166686 Free PMC article.
-
A novel role for Dun1 in the regulation of origin firing upon hyper-acetylation of H3K56.PLoS Genet. 2021 Feb 18;17(2):e1009391. doi: 10.1371/journal.pgen.1009391. eCollection 2021 Feb. PLoS Genet. 2021. PMID: 33600490 Free PMC article.
-
A genetic interaction map centered on cohesin reveals auxiliary factors involved in sister chromatid cohesion in S. cerevisiae.J Cell Sci. 2020 May 22;133(10):jcs237628. doi: 10.1242/jcs.237628. J Cell Sci. 2020. PMID: 32299836 Free PMC article.
-
E3 ubiquitin ligase Bre1 couples sister chromatid cohesion establishment to DNA replication in Saccharomyces cerevisiae.Elife. 2017 Oct 23;6:e28231. doi: 10.7554/eLife.28231. Elife. 2017. PMID: 29058668 Free PMC article.
-
Rtt101-Mms1-Mms22 coordinates replication-coupled sister chromatid cohesion and nucleosome assembly.EMBO Rep. 2017 Aug;18(8):1294-1305. doi: 10.15252/embr.201643807. Epub 2017 Jun 14. EMBO Rep. 2017. PMID: 28615292 Free PMC article.
References
-
- Aparicio, O. M., D. M. Weinstein, and S. Bell. 1997. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell 91:59-69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

