Role of outer ring carboxylates of the rat skeletal muscle sodium channel pore in proton block
- PMID: 12181282
- PMCID: PMC2290475
- DOI: 10.1113/jphysiol.2002.021014
Role of outer ring carboxylates of the rat skeletal muscle sodium channel pore in proton block
Abstract
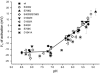
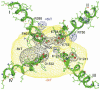
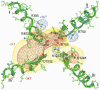
Voltage-gated Na+ current is reduced by acid solution. Protons reduce peak Na+ conductance by lowering single channel conductance and shift the voltage range of gating by neutralizing surface charges. Structure-function studies identify six carboxyls and a lysine in the channel's outer vestibule. We examined the roles of the superficial ring of carboxyls in acid block of Na(v)1.4 (the rat skeletal muscle Na+ channel isoform) by measuring the effects of their neutralization or their substitution by lysine on sensitivity to acid solutions, using the two-micropipette voltage clamp in Xenopus oocytes. Alteration of the outer ring of carboxylates had little effect on the voltage for half-activation of Na+ current, as if they are distant from the channels' voltage sensors. The mutations did not abolish proton block; rather, they all shifted the pK(a) (-log of the dissociation constant) in the acid direction. Effects of neutralization on pK(a) were not identical for different mutations, with E758Q > D1241A > D1532N > E403Q. E758K showed double the effect of E758Q, and the other lysine mutations all produced larger effects than the neutralizing mutations. Calculation of the electrostatic potential produced by these carboxylates using a pore model showed that the pK(a) values of carboxylates of Glu-403, Glu-758, and Asp-1532 are shifted to values similar to the experimentally measured pK(a). Calculations also predict the experimentally observed changes in pK(a) that result from mutational neutralization or introduction of a positive charge. We propose that proton block results from partial protonation of these outer ring carboxylates and that all of the carboxylates contribute to a composite Na+ site.
Figures




Similar articles
-
Isoform-dependent interaction of voltage-gated sodium channels with protons.J Physiol. 2006 Oct 15;576(Pt 2):493-501. doi: 10.1113/jphysiol.2006.115659. Epub 2006 Jul 27. J Physiol. 2006. PMID: 16873405 Free PMC article.
-
Sodium channel activation gating is affected by substitutions of voltage sensor positive charges in all four domains.J Gen Physiol. 1997 Oct;110(4):391-401. doi: 10.1085/jgp.110.4.391. J Gen Physiol. 1997. PMID: 9379171 Free PMC article.
-
Proton inhibition of sodium channels: mechanism of gating shifts and reduced conductance.J Membr Biol. 1997 Jan 15;155(2):121-31. doi: 10.1007/s002329900164. J Membr Biol. 1997. PMID: 9049106
-
Molecular pore structure of voltage-gated sodium and calcium channels.Braz J Med Biol Res. 1994 Dec;27(12):2781-802. Braz J Med Biol Res. 1994. PMID: 7550000 Review.
-
Voltage-gated Na channel selectivity: the role of the conserved domain III lysine residue.J Gen Physiol. 2008 Jun;131(6):523-9. doi: 10.1085/jgp.200809991. J Gen Physiol. 2008. PMID: 18504313 Free PMC article. Review. No abstract available.
Cited by
-
Historical Perspective of the Characterization of Conotoxins Targeting Voltage-Gated Sodium Channels.Mar Drugs. 2023 Mar 27;21(4):209. doi: 10.3390/md21040209. Mar Drugs. 2023. PMID: 37103349 Free PMC article. Review.
-
Sodium channels and the ionic microenvironment of breast tumours.J Physiol. 2023 May;601(9):1543-1553. doi: 10.1113/JP282306. Epub 2022 Oct 21. J Physiol. 2023. PMID: 36183245 Free PMC article.
-
Cationic Modulation of Voltage-Gated Sodium Channel (Nav1.5): Neonatal Versus Adult Splice Variants-1. Monovalent (H+) Ions.Bioelectricity. 2019 Sep 1;1(3):139-147. doi: 10.1089/bioe.2019.0012. Epub 2019 Sep 16. Bioelectricity. 2019. PMID: 34471816 Free PMC article.
-
Structural and Functional Analyses of Cone Snail Toxins.Mar Drugs. 2019 Jun 21;17(6):370. doi: 10.3390/md17060370. Mar Drugs. 2019. PMID: 31234371 Free PMC article. Review.
-
Chemogenic Subqualities of Mouthfeel.Chem Senses. 2019 May 29;44(5):281-288. doi: 10.1093/chemse/bjz016. Chem Senses. 2019. PMID: 31039245 Free PMC article. Review.
References
-
- Antosiewicz J, McCammon JA, Gilson MK. Prediction of pH-dependent properties of proteins. Journal of Molecular Biology. 1994;238:415–436. - PubMed
-
- Bashford D, Karplus M. pKa's of ionizable groups in proteins: atomic detail from a continuum electrostatic model. Biochemistry. 1990;29:10219–10225. - PubMed
-
- Benitah J-P, Balser JR, Marbán E, Tomaselli GF. Proton inhibition of sodium channels: mechanisms of gating shifts and reduced conductance. Journal of Membrane Biology. 1997;155:121–131. - PubMed
-
- Chang NS, French RJ, Lipkind GM, FoARDZZ HA, Dudley SC., Jr Predominant interactions between μ-conotoxin Arg-13 and the skeletal muscle Na+ channel localized by mutant cycle analysis. Biochemistry. 1998;37:4407–4419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources


