Contactin associates with Na+ channels and increases their functional expression
- PMID: 11567041
- PMCID: PMC6762905
- DOI: 10.1523/JNEUROSCI.21-19-07517.2001
Contactin associates with Na+ channels and increases their functional expression
Abstract
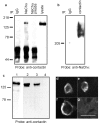
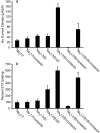
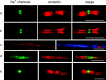
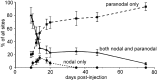
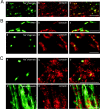
Contactin (also known as F3, F11) is a surface glycoprotein that has significant homology with the beta2 subunit of voltage-gated Na(+) channels. Contactin and Na(+) channels can be reciprocally coimmunoprecipitated from brain homogenates, indicating association within a complex. Cells cotransfected with Na(+) channel Na(v)1.2alpha and beta1 subunits and contactin have threefold to fourfold higher peak Na(+) currents than cells with Na(v)1.2alpha alone, Na(v)1.2/beta1, Na(v)1.2/contactin, or Na(v)1.2/beta1/beta2. These cells also have a correspondingly higher saxitoxin binding, suggesting an increased Na(+) channel surface membrane density. Coimmunoprecipitation of different subunits from cell lines shows that contactin interacts specifically with the beta1 subunit. In the PNS, immunocytochemical studies show a transient colocalization of contactin and Na(+) channels at new nodes of Ranvier forming during remyelination. In the CNS, there is a particularly high level of colocalization of Na(+) channels and contactin at nodes both during development and in the adult. Contactin may thus significantly influence the functional expression and distribution of Na(+) channels in neurons.
Figures


Similar articles
-
Contactin associates with sodium channel Nav1.3 in native tissues and increases channel density at the cell surface.J Neurosci. 2004 Aug 18;24(33):7387-99. doi: 10.1523/JNEUROSCI.0322-04.2004. J Neurosci. 2004. PMID: 15317864 Free PMC article.
-
Sodium channel beta1 subunit-mediated modulation of Nav1.2 currents and cell surface density is dependent on interactions with contactin and ankyrin.J Biol Chem. 2004 Apr 16;279(16):16044-9. doi: 10.1074/jbc.M400856200. Epub 2004 Feb 3. J Biol Chem. 2004. PMID: 14761957
-
Mice lacking sodium channel beta1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture.J Neurosci. 2004 Apr 21;24(16):4030-42. doi: 10.1523/JNEUROSCI.4139-03.2004. J Neurosci. 2004. PMID: 15102918 Free PMC article.
-
Nodes of Ranvier come of age.Trends Neurosci. 2002 Jan;25(1):2-5. doi: 10.1016/s0166-2236(00)02006-3. Trends Neurosci. 2002. PMID: 11801321 Review.
-
Molecular constituents of the node of Ranvier.Mol Neurobiol. 2002 Oct-Dec;26(2-3):167-82. doi: 10.1385/MN:26:2-3:167. Mol Neurobiol. 2002. PMID: 12428754 Review.
Cited by
-
Antibody-directed extracellular proximity biotinylation reveals that Contactin-1 regulates axo-axonic innervation of axon initial segments.Nat Commun. 2023 Oct 26;14(1):6797. doi: 10.1038/s41467-023-42273-8. Nat Commun. 2023. PMID: 37884508 Free PMC article.
-
A Push-Pull Mechanism Between PRRT2 and β4-subunit Differentially Regulates Membrane Exposure and Biophysical Properties of NaV1.2 Sodium Channels.Mol Neurobiol. 2023 Mar;60(3):1281-1296. doi: 10.1007/s12035-022-03112-x. Epub 2022 Nov 28. Mol Neurobiol. 2023. PMID: 36441479 Free PMC article.
-
To Stick or Not to Stick: The Multiple Roles of Cell Adhesion Molecules in Neural Circuit Assembly.Front Neurosci. 2022 Apr 28;16:889155. doi: 10.3389/fnins.2022.889155. eCollection 2022. Front Neurosci. 2022. PMID: 35573298 Free PMC article. Review.
-
The AMIGO1 adhesion protein activates Kv2.1 voltage sensors.Biophys J. 2022 Apr 19;121(8):1395-1416. doi: 10.1016/j.bpj.2022.03.020. Epub 2022 Mar 18. Biophys J. 2022. PMID: 35314141 Free PMC article.
-
Live imaging of retinotectal mapping reveals topographic map dynamics and a previously undescribed role for Contactin 2 in map sharpening.Development. 2021 Nov 15;148(22):dev199584. doi: 10.1242/dev.199584. Epub 2021 Nov 15. Development. 2021. PMID: 34698769 Free PMC article.
References
-
- Bennett V, Lambert S. Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels. J Neurocytol. 1999;28:303–318. - PubMed
-
- Berglund EO, Murai KK, Fredette B, Sekerkova G, Marturano B, Weber L, Mugnaini E, Ranscht B. Ataxia and abnormal cerebellar microorganization in mice with ablated contactin gene expression. Neuron. 1999;24:739–750. - PubMed
-
- Berglund EO, Boyle MET, Murai KK, Peles E, Weber L, Ranscht B. Contactin regulates axon–Schwann cell interactions at the paranode in myelinated peripheral nerve. Soc Neurosci Abstr. 2000;26:1081.
-
- Brummendorf T, Wolff JM, Frank R, Rathjen FG. Neural cell recognition molecule F11: homology with fibronectin type III and immunoglobulin type C domains. Neuron. 1989;2:1351–1361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

