Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2
- PMID: 11208866
- PMCID: PMC2193345
- DOI: 10.1084/jem.193.2.255
Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2
Abstract
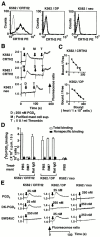
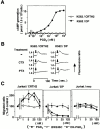
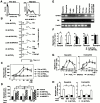
Prostaglandin (PG)D2, which has long been implicated in allergic diseases, is currently considered to elicit its biological actions through the DP receptor (DP). Involvement of DP in the formation of allergic asthma was recently demonstrated with DP-deficient mice. However, proinflammatory functions of PGD2 cannot be explained by DP alone. We show here that a seven-transmembrane receptor, CRTH2, which is preferentially expressed in T helper type 2 (Th2) cells, eosinophils, and basophils in humans, serves as the novel receptor for PGD2. In response to PGD2, CRTH2 induces intracellular Ca2+mobilization and chemotaxis in Th2 cells in a Galphai-dependent manner. In addition, CRTH2, but not DP, mediates PGD2-dependent cell migration of blood eosinophils and basophils. Thus, PGD2 is likely involved in multiple aspects of allergic inflammation through its dual receptor systems, DP and CRTH2.
Figures
Similar articles
-
Delta12-prostaglandin D2 is a potent and selective CRTH2 receptor agonist and causes activation of human eosinophils and Th2 lymphocytes.Prostaglandins Other Lipid Mediat. 2005 Jan;75(1-4):153-67. doi: 10.1016/j.prostaglandins.2004.11.003. Prostaglandins Other Lipid Mediat. 2005. PMID: 15789622
-
Differential modulation of human basophil functions through prostaglandin D2 receptors DP and chemoattractant receptor-homologous molecule expressed on Th2 cells/DP2.Clin Exp Allergy. 2004 Aug;34(8):1283-90. doi: 10.1111/j.1365-2222.2004.02027.x. Clin Exp Allergy. 2004. PMID: 15298571
-
Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells.J Immunol. 2005 Nov 15;175(10):6531-6. doi: 10.4049/jimmunol.175.10.6531. J Immunol. 2005. PMID: 16272307
-
Antagonists of the prostaglandin D2 receptor CRTH2.Drug News Perspect. 2008 Jul-Aug;21(6):317-22. doi: 10.1358/dnp.2008.21.6.1246831. Drug News Perspect. 2008. PMID: 18836589 Review.
-
[Prostaglandin D2 in allergy: PGD2 has dual receptor systems].Nihon Yakurigaku Zasshi. 2004 Jan;123(1):15-22. doi: 10.1254/fpj.123.15. Nihon Yakurigaku Zasshi. 2004. PMID: 14695454 Review. Japanese.
Cited by
-
Serum metabolomics analysis reveals potential biomarkers of penicillins-induced fatal anaphylactic shock in rats.Sci Rep. 2024 Oct 9;14(1):23534. doi: 10.1038/s41598-024-74623-x. Sci Rep. 2024. PMID: 39384950 Free PMC article.
-
Spatiotemporally distinct roles of cyclooxygenase-1 and cyclooxygenase-2 at fetomaternal interface in mice.JCI Insight. 2024 Aug 27;9(19):e181865. doi: 10.1172/jci.insight.181865. JCI Insight. 2024. PMID: 39377223 Free PMC article.
-
Endogenous PGD2 acting on DP2 receptor counter regulates Schistosoma mansoni infection-driven hepatic granulomatous fibrosis.PLoS Pathog. 2024 Aug 22;20(8):e1011812. doi: 10.1371/journal.ppat.1011812. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39173086 Free PMC article.
-
The cGAS-STING pathway in COPD: targeting its role and therapeutic potential.Respir Res. 2024 Aug 7;25(1):302. doi: 10.1186/s12931-024-02915-x. Respir Res. 2024. PMID: 39113033 Free PMC article. Review.
-
Uncovering novel mechanisms of chitinase-3-like protein 1 in driving inflammation-associated cancers.Cancer Cell Int. 2024 Jul 27;24(1):268. doi: 10.1186/s12935-024-03425-y. Cancer Cell Int. 2024. PMID: 39068486 Free PMC article. Review.
References
-
- Murphy P.M. The molecular biology of leukocyte chemoattractant receptors. Annu. Rev. Immunol. 1994;12:593–633. - PubMed
-
- Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigrationthe multistep paradigm. Cell. 1994;76:301–314. - PubMed
-
- Robinson D.S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A.M., Corrigan C., Durham S.R., Kay A.B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 1992;326:298–304. - PubMed
-
- Nagata K., Tanaka K., Ogawa K., Kemmotsu K., Imai T., Yoshie O., Abe H., Tada K., Nakamura M., Sugamura K. Selective expression of a novel surface molecule by human Th2 cells in vivo. J. Immunol. 1999;162:1278–1286. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous


