Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers
- PMID: 10817726
- PMCID: PMC89930
- DOI: 10.1128/AAC.44.6.1667-1673.2000
Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers
Abstract
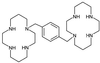
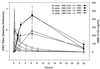
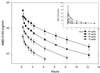
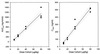
AMD-3100, a bicyclam, is a novel agent that uniquely inhibits the entry of human immunodeficiency virus type 1 (HIV-1) into CD4(+) T cells via selective blockade of the chemokine CXCR-4 receptor. Twelve healthy volunteers were given AMD-3100 as a single 15-min intravenous infusion at 10, 20, 40, or 80 microg/kg. Five subjects also received a single subcutaneous injection of AMD-3100 (40 or 80 microg/kg). Three subjects received two escalating oral doses each (80 and 160 microg/kg). All subjects tolerated their dose(s) well without any grade 2 toxicity or dose adjustment. Six subjects experienced mild, transient symptoms, primarily gastrointestinal in nature and not dose related. All subjects experienced a dose-related elevation of the white blood cell count, from 1.5 to 3.1 times the baseline, which returned to the baseline 24 h after dosing. AMD-3100 demonstrated dose proportionality for the maximum drug concentration in serum (C(max)) and the area under the concentration-time curve from 0 h to infinity (AUC(0-infinity)) over the entire dose range. At the highest intravenous dose (80 microg/kg), the median C(max) was 515 (range, 470 to 521) ng/ml and the AUC(0-infinity) was 1,044 (range, 980 to 1,403) ng-h/ml. The median systemic absorption after subcutaneous dosing was 87% (range, 67 to 106%). No drug was detectable in the blood following oral dosing. Using a two-compartment model, the median pharmacokinetic parameter estimates (ranges) were as follows: volume of distribution, 0.34 (0. 27 to 0.36) liter/kg; clearance, 1.30 (0.97 to 1.34) liters/h; elimination half-life, 3.6 (3.5 to 4.9) h. After a single, well-tolerated intravenous dose of AMD-3100, concentrations were sustained for 12 h above the in vitro antiretroviral 90% inhibitory concentrations and for 8 h above antiviral concentrations identified in the SCID-hu Thy/Liv mouse model of HIV infection.
Figures
Similar articles
-
Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection.J Acquir Immune Defic Syndr. 2004 Oct 1;37(2):1253-62. doi: 10.1097/01.qai.0000137371.80695.ef. J Acquir Immune Defic Syndr. 2004. PMID: 15385732 Clinical Trial.
-
Multiple-dose escalation study of the safety, pharmacokinetics, and biologic activity of oral AMD070, a selective CXCR4 receptor inhibitor, in human subjects.Antimicrob Agents Chemother. 2007 Jul;51(7):2351-8. doi: 10.1128/AAC.00013-07. Epub 2007 Apr 23. Antimicrob Agents Chemother. 2007. PMID: 17452489 Free PMC article. Clinical Trial.
-
Effect of low-dose ritonavir on the pharmacokinetics of the CXCR4 antagonist AMD070 in healthy volunteers.Antimicrob Agents Chemother. 2008 May;52(5):1630-4. doi: 10.1128/AAC.01460-07. Epub 2008 Feb 19. Antimicrob Agents Chemother. 2008. PMID: 18285477 Free PMC article. Clinical Trial.
-
Recent patents regarding the discovery of small molecule CXCR4 antagonists.Expert Opin Ther Pat. 2009 Jan;19(1):23-38. doi: 10.1517/13543770802553483. Expert Opin Ther Pat. 2009. PMID: 19441896 Review.
-
Clinical pharmacokinetics of lamivudine.Clin Pharmacokinet. 1999 Jan;36(1):41-66. doi: 10.2165/00003088-199936010-00004. Clin Pharmacokinet. 1999. PMID: 9989342 Review.
Cited by
-
CXCR4 as a therapeutic target in acute myeloid leukemia.Leukemia. 2024 Nov;38(11):2303-2317. doi: 10.1038/s41375-024-02326-3. Epub 2024 Sep 11. Leukemia. 2024. PMID: 39261603 Review.
-
Allosteric modulation of the CXCR4:CXCL12 axis by targeting receptor nanoclustering via the TMV-TMVI domain.Elife. 2024 Sep 9;13:RP93968. doi: 10.7554/eLife.93968. Elife. 2024. PMID: 39248648 Free PMC article.
-
Potentiating effect of AMD3100 on bone morphogenetic protein-2 induced bone regeneration.Maxillofac Plast Reconstr Surg. 2024 Jun 17;46(1):22. doi: 10.1186/s40902-024-00431-y. Maxillofac Plast Reconstr Surg. 2024. PMID: 38884872 Free PMC article.
-
Selected Milestones in Antiviral Drug Development.Viruses. 2024 Jan 23;16(2):169. doi: 10.3390/v16020169. Viruses. 2024. PMID: 38399945 Free PMC article. Review.
-
An evidence-based and risk-adapted GSF versus GSF plus plerixafor mobilization strategy to obtain a sufficient CD34+ cell yield in the harvest for autologous stem cell transplants.Transl Oncol. 2024 Jan;39:101811. doi: 10.1016/j.tranon.2023.101811. Epub 2023 Oct 31. Transl Oncol. 2024. PMID: 38235620 Free PMC article.
References
-
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials


