Calcium-, voltage- and osmotic stress-sensitive currents in Xenopus oocytes and their relationship to single mechanically gated channels
- PMID: 10673546
- PMCID: PMC2269778
- DOI: 10.1111/j.1469-7793.2000.t01-2-00083.x
Calcium-, voltage- and osmotic stress-sensitive currents in Xenopus oocytes and their relationship to single mechanically gated channels
Abstract
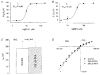
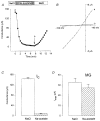
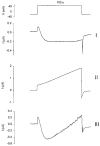
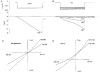
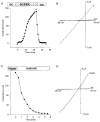
1. Patch recordings from Xenopus oocytes indicated that mechanically gated (MG) channels are expressed at a uniform surface density ( approximately 1 channel microm-2) with an estimated > 3 x 106 MG channels per oocyte that could generate microamps of current at +/-50 mV. 2. Removal of external Ca2+ induced a membrane conductance that differed from MG channels in ion selectivity, pharmacology and sensitivity to connexion-38. 3. Depolarization to +50 mV activated a Na+-selective, a Cl--selective and a non-selective conductance. Hyperpolarization to -150 mV activated a non-selective conductance. None of these conductances appeared to be mediated by MG channels. 4. Hypotonicity (25 %) failed to evoke any change in membrane conductance in the majority of defolliculated oocytes. Hypertonicity (200 %) evoked a large non-selective (PK /PCl approximately 1) membrane conductance that was not blocked by 100 microM Gd3+. 5. Although the above stimuli could activate a variety of whole-oocyte conductances, including three novel conductances, they did not involve MG channel activation. Possible mechanisms underlying the discrepancy between observed conductances and those anticipated from patch-clamp studies are discussed.
Figures
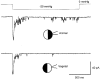

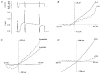
 ) in the bath and pipette solutions (data from 6 patches in each condition using paired pipettes from single pulls). NaCl solution (m
) in the bath and pipette solutions (data from 6 patches in each condition using paired pipettes from single pulls). NaCl solution (m



Similar articles
-
The ion selectivity of a membrane conductance inactivated by extracellular calcium in Xenopus oocytes.J Physiol. 1998 May 1;508 ( Pt 3)(Pt 3):763-76. doi: 10.1111/j.1469-7793.1998.763bp.x. J Physiol. 1998. PMID: 9518731 Free PMC article.
-
Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels.Pflugers Arch. 1986 Dec;407(6):577-88. doi: 10.1007/BF00582635. Pflugers Arch. 1986. PMID: 2432468
-
Electrophysiological characteristics of rat gustatory cyclic nucleotide--gated channel expressed in Xenopus oocytes.J Neurophysiol. 2001 Jun;85(6):2335-49. doi: 10.1152/jn.2001.85.6.2335. J Neurophysiol. 2001. PMID: 11387380
-
Studying the mechanosensitivity of voltage-gated channels using oocyte patches.Methods Mol Biol. 2006;322:315-29. doi: 10.1007/978-1-59745-000-3_22. Methods Mol Biol. 2006. PMID: 16739733 Review.
-
High throughput electrophysiology with Xenopus oocytes.Comb Chem High Throughput Screen. 2009 Jan;12(1):38-50. doi: 10.2174/138620709787047975. Comb Chem High Throughput Screen. 2009. PMID: 19149490 Free PMC article. Review.
Cited by
-
A hyperpolarization-activated ion current of amphibian oocytes.Pflugers Arch. 2013 Aug;465(8):1087-99. doi: 10.1007/s00424-013-1231-2. Epub 2013 Feb 26. Pflugers Arch. 2013. PMID: 23440457
-
Piezo1: properties of a cation selective mechanical channel.Channels (Austin). 2012 Jul-Aug;6(4):214-9. doi: 10.4161/chan.21050. Epub 2012 Jul 1. Channels (Austin). 2012. PMID: 22790400 Free PMC article. Review.
-
Wild-type and brachyolmia-causing mutant TRPV4 channels respond directly to stretch force.J Biol Chem. 2010 Aug 27;285(35):27176-27181. doi: 10.1074/jbc.M110.143370. Epub 2010 Jul 6. J Biol Chem. 2010. PMID: 20605796 Free PMC article.
-
Twenty odd years of stretch-sensitive channels.Pflugers Arch. 2006 Dec;453(3):333-51. doi: 10.1007/s00424-006-0131-0. Epub 2006 Sep 21. Pflugers Arch. 2006. PMID: 17021800 Review.
-
Mechanosensitive cation channels in human leukaemia cells: calcium permeation and blocking effect.J Physiol. 2002 May 15;541(Pt 1):81-90. doi: 10.1113/jphysiol.2001.015222. J Physiol. 2002. PMID: 12015421 Free PMC article.
References
-
- Achard J-M, Bubien JK, Benos DJ, Warnock DG. Stretch modulates amiloride sensitivity and cation selectivity of sodium channels in human B-lymphocytes. American Journal of Physiology. 1996;270:C224–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous


