Nuclear and cell membrane effects contribute independently to the induction of apoptosis in human cells exposed to UVB radiation
- PMID: 10393932
- PMCID: PMC22172
- DOI: 10.1073/pnas.96.14.7974
Nuclear and cell membrane effects contribute independently to the induction of apoptosis in human cells exposed to UVB radiation
Abstract
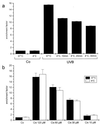
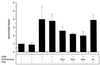
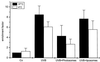
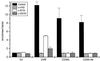
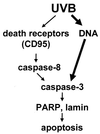
UVB-induced DNA damage is a crucial event in UVB-mediated apoptosis. On the other hand, UVB directly activates death receptors on the cell surface including CD95, implying that UVB-induced apoptosis can be initiated at the cell membrane through death receptor clustering. This study was performed to measure the relative contribution of nuclear and membrane effects in UVB-induced apoptosis of the human epithelial cell line HeLa. UVB-mediated DNA damage can be reduced by treating cells with liposomes containing the repair enzyme photolyase followed by exposure to photoreactivating light. Addition of photolyase followed by photoreactivation after UVB reduced the apoptosis rate significantly, whereas empty liposomes had no effect. Likewise, photoreactivating treatment did not affect apoptosis induced by the ligand of CD95, CD95L. UVB exposure at 4 degrees C, which prevents CD95 clustering, also reduced the apoptosis rate, but to a lesser extent. When cells were exposed to UVB at 4 degrees C and treated with photolyase plus photoreactivating light, UVB-induced apoptosis was almost completely prevented. Inhibition of caspase-3, a downstream protease in the CD95 signaling pathway, blocked both CD95L and UVB-induced apoptosis, whereas blockage of caspase-8, the most proximal caspase, inhibited CD95L-mediated apoptosis completely, but UVB-induced apoptosis only partially. Although according to these data nuclear effects seem to be slightly more effective in mediating UVB-induced apoptosis than membrane events, both are necessary for the complete apoptotic response. Thus, this study shows that nuclear and membrane effects are not mutually exclusive and that both components contribute independently to a complete response to UVB.
Figures


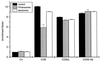
Similar articles
-
Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L.J Cell Biol. 1998 Jan 12;140(1):171-82. doi: 10.1083/jcb.140.1.171. J Cell Biol. 1998. PMID: 9425165 Free PMC article.
-
Ultraviolet radiation-induced interleukin 6 release in HeLa cells is mediated via membrane events in a DNA damage-independent way.J Biol Chem. 2000 May 19;275(20):15060-6. doi: 10.1074/jbc.M910113199. J Biol Chem. 2000. PMID: 10748190
-
DNA damage, death receptor activation and reactive oxygen species contribute to ultraviolet radiation-induced apoptosis in an essential and independent way.Oncogene. 2002 Aug 29;21(38):5844-51. doi: 10.1038/sj.onc.1205743. Oncogene. 2002. PMID: 12185583
-
Distinct intracellular signaling in tumor necrosis factor-related apoptosis-inducing ligand- and CD95 ligand-mediated apoptosis.J Biol Chem. 2002 Jul 5;277(27):24631-7. doi: 10.1074/jbc.M111572200. Epub 2002 Apr 29. J Biol Chem. 2002. PMID: 11980895
-
The CD95(APO-1/Fas)/CD95L system.Toxicol Lett. 1998 Dec 28;102-103:131-7. doi: 10.1016/s0378-4274(98)00297-5. Toxicol Lett. 1998. PMID: 10022244 Review.
Cited by
-
Downregulation of SMAD4 protects HaCaT cells against UVB-induced damage and oxidative stress through the activation of EMT.Photochem Photobiol Sci. 2024 Jun;23(6):1051-1065. doi: 10.1007/s43630-024-00574-x. Epub 2024 Apr 29. Photochem Photobiol Sci. 2024. PMID: 38684635
-
Smart Drug-Delivery System of Upconversion Nanoparticles Coated with Mesoporous Silica for Controlled Release.Pharmaceutics. 2022 Dec 27;15(1):89. doi: 10.3390/pharmaceutics15010089. Pharmaceutics. 2022. PMID: 36678718 Free PMC article.
-
A review on cell damage, viability, and functionality during 3D bioprinting.Mil Med Res. 2022 Dec 16;9(1):70. doi: 10.1186/s40779-022-00429-5. Mil Med Res. 2022. PMID: 36522661 Free PMC article. Review.
-
Development of Second Generation Activity-Based Chemical Probes for Sirtuins.Molecules. 2020 Dec 22;26(1):11. doi: 10.3390/molecules26010011. Molecules. 2020. PMID: 33375102 Free PMC article.
-
Application of adipose-derived stem cells in photoaging: basic science and literature review.Stem Cell Res Ther. 2020 Nov 23;11(1):491. doi: 10.1186/s13287-020-01994-z. Stem Cell Res Ther. 2020. PMID: 33225962 Free PMC article. Review.
References
-
- Chapman R S, Cooper K D, DeFabo E C, Frederich J E, Gelatt K N, Hammond S P, Hersey P, Koren H S, Ley R D, Noonan F, et al. Photochem Photobiol. 1995;61:223–247. - PubMed
-
- De Gruijl F R, Sterenborg H J, Forbes P D, Davies R E, Cole C, Kelfkens G, van Weelden H, Slaper H, van der Leun J C. Cancer Res. 1993;53:53–60. - PubMed
-
- Fisher G J, Datta S C, Talwar H S, Wang Z Q, Varani J, Kang S, Voorhees J J. Nature (London) 1996;379:335–339. - PubMed
-
- Herrlich P, Ponta H, Rahmsdorf H J. Rev Physiol Biochem Pharmacol. 1992;119:187–223. - PubMed
-
- Herrlich P, Rahmsdorf H J. Curr Opin Cell Biol. 1994;6:425–431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials


