Fanconi anemia proteins FANCA, FANCC, and FANCG/XRCC9 interact in a functional nuclear complex
- PMID: 10373536
- PMCID: PMC84285
- DOI: 10.1128/MCB.19.7.4866
Fanconi anemia proteins FANCA, FANCC, and FANCG/XRCC9 interact in a functional nuclear complex
Abstract
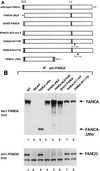
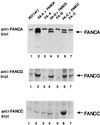
Fanconi anemia (FA) is an autosomal recessive cancer susceptibility syndrome with at least eight complementation groups (A to H). Three FA genes, corresponding to complementation groups A, C, and G, have been cloned, but their cellular function remains unknown. We have previously demonstrated that the FANCA and FANCC proteins interact and form a nuclear complex in normal cells, suggesting that the proteins cooperate in a nuclear function. In this report, we demonstrate that the recently cloned FANCG/XRCC9 protein is required for binding of the FANCA and FANCC proteins. Moreover, the FANCG protein is a component of a nuclear protein complex containing FANCA and FANCC. The amino-terminal region of the FANCA protein is required for FANCG binding, FANCC binding, nuclear localization, and functional activity of the complex. Our results demonstrate that the three cloned FA proteins cooperate in a large multisubunit complex. Disruption of this complex results in the specific cellular and clinical phenotype common to most FA complementation groups.
Figures




Similar articles
-
Carboxy terminal region of the Fanconi anemia protein, FANCG/XRCC9, is required for functional activity.Blood. 2000 Sep 1;96(5):1625-32. Blood. 2000. PMID: 10961856
-
The fanconi anemia proteins FANCA and FANCG stabilize each other and promote the nuclear accumulation of the Fanconi anemia complex.Blood. 2000 Nov 1;96(9):3224-30. Blood. 2000. PMID: 11050007
-
The Fanconi anemia protein FANCF forms a nuclear complex with FANCA, FANCC and FANCG.Hum Mol Genet. 2000 Nov 1;9(18):2665-74. doi: 10.1093/hmg/9.18.2665. Hum Mol Genet. 2000. PMID: 11063725
-
Molecular pathogenesis of fanconi anemia.Int J Hematol. 2002 Feb;75(2):123-8. doi: 10.1007/BF02982016. Int J Hematol. 2002. PMID: 11939257 Review.
-
[Molecular basis of Fanconi's anemia].Klin Padiatr. 1999 Jul-Aug;211(4):192-7. doi: 10.1055/s-2008-1043786. Klin Padiatr. 1999. PMID: 10472548 Review. German.
Cited by
-
The splicing factor CCAR1 regulates the Fanconi anemia/BRCA pathway.Mol Cell. 2024 Jul 25;84(14):2618-2633.e10. doi: 10.1016/j.molcel.2024.06.031. Epub 2024 Jul 17. Mol Cell. 2024. PMID: 39025073 Free PMC article.
-
A C57BL/6J Fancg-KO Mouse Model Generated by CRISPR/Cas9 Partially Captures the Human Phenotype.Int J Mol Sci. 2023 Jul 5;24(13):11129. doi: 10.3390/ijms241311129. Int J Mol Sci. 2023. PMID: 37446306 Free PMC article.
-
SIK2 kinase synthetic lethality is driven by spindle assembly defects in FANCA-deficient cells.Mol Oncol. 2022 Feb;16(4):860-884. doi: 10.1002/1878-0261.13027. Epub 2021 Jun 28. Mol Oncol. 2022. PMID: 34058059 Free PMC article.
-
Myelodysplastic Syndrome, Acute Myeloid Leukemia, and Cancer Surveillance in Fanconi Anemia.Hematol Oncol Clin North Am. 2018 Aug;32(4):657-668. doi: 10.1016/j.hoc.2018.04.002. Hematol Oncol Clin North Am. 2018. PMID: 30047418 Free PMC article. Review.
-
A germline FANCA alteration that is associated with increased sensitivity to DNA damaging agents.Cold Spring Harb Mol Case Stud. 2017 Sep;3(5):a001487. doi: 10.1101/mcs.a001487. Epub 2017 May 3. Cold Spring Harb Mol Case Stud. 2017. PMID: 28864460 Free PMC article.
References
-
- Auerbach A D, Buchwald M, Joenje H. Fanconi anemia. In: Vogelstein B, Kinzler K W, editors. The genetic basis of human cancer. New York, N.Y: McGraw Hill; 1997.
-
- Baumann P, West S C. Role of the human RAD51 protein in homologous recombination and double-stranded break repair. Trends Biochem Sci. 1998;23:247–251. - PubMed
-
- Buchwald M. Complementation groups: one or more per gene. Nat Genet. 1995;11:228–230. - PubMed
-
- Chu G. Double strand break repair. J Biol Chem. 1997;272:24097–24100. - PubMed
-
- Cumming R C, Liu J M, Youssoufian H, Buchwald M. Suppression of apoptosis in hematopoietic factor-dependent progenitor cell lines by expression of the FAC gene. Blood. 1996;88:4558–4567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

