Abstract
Background
This review is one of six looking at the primary medical management options for patients with chronic rhinosinusitis.
Chronic rhinosinusitis is common and is characterised by inflammation of the lining of the nose and paranasal sinuses leading to nasal blockage, nasal discharge, facial pressure/pain and loss of sense of smell. The condition can occur with or without nasal polyps. Systemic and topical antibiotics are used with the aim of eliminating infection in the short term (and some to reduce inflammation in the long term), in order to normalise nasal mucus and improve symptoms.
Objectives
To assess the effects of systemic and topical antibiotics in people with chronic rhinosinusitis.
Search methods
The Cochrane ENT Information Specialist searched the Cochrane ENT Trials Register; CENTRAL (2015, Issue 8); MEDLINE; EMBASE; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 29 September 2015.
Selection criteria
Randomised controlled trials (RCTs) with a follow‐up period of at least three months comparing systemic or topical antibiotic treatment to (a) placebo or (b) no treatment or (c) other pharmacological interventions.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. Our primary outcomes were disease‐specific health‐related quality of life (HRQL), patient‐reported disease severity and the commonest adverse event ‐ gastrointestinal disturbance. Secondary outcomes included general HRQL, endoscopic nasal polyp score, computerised tomography (CT) scan score and the adverse events of suspected allergic reaction (rash or skin irritation) and anaphylaxis or other very serious reactions. We used GRADE to assess the quality of the evidence for each outcome; this is indicated in italics.
Main results
We included five RCTs (293 participants), all of which compared systemic antibiotics with placebo or another pharmacological intervention.
The varying study characteristics made comparison difficult. Four studies recruited only adults and one only children. Three used macrolide, one tetracycline and one a cephalosporin‐type antibiotic. Three recruited only patients with chronic rhinosinusitis without nasal polyps, one recruited patients with chronic rhinosinusitis with nasal polyps and one had a mixed population. Three followed up patients for 10 to 12 weeks after treatment had finished.
Systemic antibiotics versus placebo
Three studies compared antibiotics with placebo (176 participants).
One study (64 participants, without polyps) reported disease‐specific HRQL using the SNOT‐20 (0 to 5, 0 = best quality of life). At the end of treatment (three months) the SNOT‐20 score was lower in the group receiving macrolide antibiotics than the placebo group (mean difference (MD) ‐0.54 points, 95% confidence interval (CI) ‐0.98 to ‐0.10), corresponding to a moderate effect size favouring antibiotics (moderate quality evidence). Three months after treatment, it is uncertain if there was a difference between groups.
One study (33 participants, with polyps) provided information on gastrointestinal disturbances and suspected allergic reaction (rash or skin irritation) after a short course of tetracycline antibiotic compared with placebo. We are very uncertain if antibiotics were associated with an increase in gastrointestinal disturbances (risk ratio (RR) 1.36, 95% CI 0.22 to 8.50) or skin irritation (RR 6.67, 95% CI 0.34 to 128.86) (very low quality evidence).
Systemic antibiotics plus saline irrigation and intranasal corticosteroids versus placebo plus saline irrigation and intranasal corticosteroids
One study (60 participants, some with and some without polyps) compared a three‐month course of macrolide antibiotic with placebo; all participants also used saline irrigation and 70% used intranasal corticosteroids. Disease‐specific HRQL was reported using SNOT‐22 (0 to 110, 0 = best quality of life). Data were difficult to interpret (highly skewed and baseline imbalances) and it is unclear if there was an important difference at any time point (low quality evidence). To assess patient‐reported disease severity participants rated the effect of treatment on a five‐point scale (‐2 for "desperately worse" to 2 for "cured") at the end of treatment (three months). For improvement in symptoms there was no difference between the antibiotics and placebo groups; the RR was 1.50 (95% CI 0.81 to 2.79; very low quality evidence), although there were also slightly more people who felt worse after treatment in the antibiotics group. There was no demonstrable difference in the rate of gastrointestinal disturbances between the groups (RR 1.07, 95% CI 0.16 to 7.10). General HRQL was measured using the SF‐36. The authors stated that there was no difference between groups at the end of treatment (12 weeks) or two weeks later.
Systemic antibiotics versus intranasal corticosteroids
One study (43 participants, without polyps) compared a three‐month course of macrolide antibiotic with intranasal corticosteroids. Patient‐reported disease severity was assessed using a composite symptom score (0 to 40; 0 = no symptoms). It is very uncertain if there was a difference as patient‐reported disease severity was similar between groups (MD ‐0.32, 95% CI ‐2.11 to 1.47; low quality evidence).
Systemic antibiotics versus oral corticosteroids
One study (28 participants, with polyps) compared a short course of tetracycline antibiotic (unclear duration, ˜20 days) with a 20‐day course of oral corticosteroids. We were unable to extract data on any of the primary efficacy outcomes. It is uncertain if there was a difference ingastrointestinal disturbances (RR 1.00, 95% CI 0.16 to 6.14) or skin irritation (RR 2.00, 95% CI 0.20 to 19.62) as the results for these outcomes were similar between groups (very low quality evidence).
Authors' conclusions
We found very little evidence that systemic antibiotics are effective in patients with chronic rhinosinusitis. We did find moderate quality evidence of a modest improvement in disease‐specific quality of life in adults with chronic rhinosinusitis without polyps receiving three months of a macrolide antibiotic. The size of improvement was moderate (0.5 points on a five‐point scale) and only seen at the end of the three‐month treatment; by three months later no difference was found.
Despite a general understanding that antibiotics can be associated with adverse effects, including gastrointestinal disturbances, the results in this review were very uncertain because the studies were small and few events were reported.
No RCTs of topical antibiotics met the inclusion criteria.
More research in this area, particularly evaluating longer‐term outcomes and adverse effects, is required.
Plain language summary
Systemic and topical antibiotics for chronic rhinosinusitis
Review question
We reviewed the evidence for the benefits and harms of systemic (given by mouth) or topical (given by nose) antibiotics for people with chronic rhinosinusitis.
Background
Chronic rhinosinusitis is a common condition that is defined as inflammation of the nose and paranasal sinuses (a group of air‐filled spaces behind the nose, eyes and cheeks). Patients experience at least two or more of the following symptoms for at least 12 weeks: blocked nose, discharge from their nose or runny nose, pain or pressure in their face and/or a reduced sense of smell (hyposmia). Some people will also have nasal polyps, which are grape‐like swellings of the normal nasal lining inside the nasal passage and sinuses.
Study characteristics
We included five randomised controlled trials (RCTs) with a total of 293 participants. The studies were small (43 to 79 participants). Four recruited adults and the fifth children. Three studies only included people with chronic rhinosinusitis without nasal polyps, one a mix of people with and without polyps and the remaining study only people with polyps. All used different types of oral antibiotics; none looked at topical antibiotics. The antibiotics were given to patients as either antimicrobial or anti‐inflammatory agents and for different lengths of time, although in all cases we were able to look at the outcomes after three months. Antibiotics were compared with placebo, with intranasal (in the nose) steroids or with oral steroids. One study used antibiotics as an additional treatment, on top of nasal saline irrigation and most people also took intranasal steroids in this study.
Key results and quality of the evidence
When compared to a placebo (three studies), there was moderate quality evidence in one study that there may be an improvement in disease‐specific health‐related quality of life (HRQL) with oral antibiotics in people with chronic rhinosinusitis (without polyps) at the end of treatment (three months), but it is unclear if HRQL was still improved three months later. There may have been an increase in gastrointestinal disturbances and suspected allergic reaction (rash or skin irritation) with antibiotics but we are very uncertain and the quality of the evidence is very low.
Antibiotics were used alongside nasal saline irrigation and intranasal steroids (compared to placebo plus the same) in one study. It is not clear if there was an important difference in disease‐specific HRQL after treatment (three months) or at three months after treatment was completed (low quality evidence). There may have been more people in the antibiotics group who felt they had 'improved' at the end of treatment, but there were also people who had worse symptoms in both groups (very low quality evidence). It is very uncertain if there was a difference in gastrointestinal disturbances between groups.
When compared with intranasal steroids in people with chronic rhinosinusitis (without polyps), it was very uncertain if there was a difference in disease severity (using a combined score for four different symptoms) between the antibiotics and intranasal steroids groups in one study (low quality evidence). No information was given about adverse events.
The one study that compared antibiotics with oral steroids (in people with chronic rhinosinusitis with polyps) did not present any effectiveness results that we could use. It was uncertain if there was any difference in gastrointestinal disturbances or skin irritation in the antibiotics group (very low quality evidence).
There were no reports of any serious adverse effects in any of the studies.
Conclusions
We found very little evidence that oral antibiotics are effective in patients with chronic rhinosinusitis. We did find moderate quality evidence of a modest improvement in disease‐specific quality of life in adults with chronic rhinosinusitis without polyps receiving three months of a macrolide antibiotic. The size of the improvement was moderate (0.5 points on a five‐point scale) and only seen at the end of the three‐month treatment; by three months later no difference was found.
Despite a general understanding that antibiotics can be associated with adverse effects, including gastrointestinal disturbances, the results in this review were very uncertain because the studies were small and few events were reported.
More research in this area, particularly evaluating longer‐term outcomes and adverse effects, is required.
Summary of findings
Background
Description of the condition
Chronic rhinosinusitis is defined as inflammation of the nose and paranasal sinuses. It is characterised by two or more symptoms, one of which must be nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip). The other possible symptoms include facial pain/pressure, reduction or loss of sense of smell (in adults) or cough (in children). Symptoms must have continued for at least 12 weeks. In addition, people must have either mucosal changes within the ostiomeatal complex or sinuses (or both) as evidenced by a computerised tomography (CT) scan and/or endoscopic signs of at least one of the following: nasal polyps, mucopurulent discharge primarily from the middle meatus or oedema/mucosal obstruction primarily in the middle meatus (EPOS 2012).
Chronic rhinosinusitis represents a common source of ill health; 11% of UK adults reported chronic rhinosinusitis symptoms in a worldwide population study (Hastan 2011). Symptoms, including nasal obstruction, nasal discharge, facial pain, anosmia and sleep disturbance, have a major impact on quality of life, reportedly greater in several domains of the SF‐36 than angina or chronic respiratory disease (Gliklich 1995). Acute exacerbations, inadequate symptom control and respiratory disease exacerbation are common. Complications are rare, but may include visual impairment and intracranial infection.
Two major phenotypes of chronic rhinosinusitis have been identified based on the presence or absence of nasal polyps on examination. Nasal polyps are tumour‐like hyperplastic swellings of the nasal mucosa, most commonly originating from within the ostiomeatal complex (Larsen 2004). Chronic rhinosinusitis with nasal polyps (CRSwNP) is diagnosed when polyps are seen (on direct or endoscopic examination) bilaterally in the middle meatus. The acronym CRSsNP is used for the condition in which no polyps are present.
Although the aetiology of chronic rhinosinusitis is not fully understood, it may involve abnormalities in the host response to irritants, commensal and pathogenic organisms and allergens, obstruction of sinus drainage pathways, abnormalities of normal mucociliary function, loss of the normal mucosal barrier or infection. Two typical profiles may be observed with respect to inflammatory mediators; in eosinophilic chronic rhinosinusitis, which is typically associated with nasal polyps, high levels of eosinophils, immunoglobulin E (IgE) and interleukin (IL)‐5 may be found, while in neutrophilic chronic rhinosinusitis, more often associated with chronic rhinosinusitis without polyps, neutrophils predominate, with elevated interferon (IFN) gamma, IL‐8 and tumour necrosis factor (TNF) (EPOS 2012).
While treatment decisions should be made based on an understanding of the patient's chronic rhinosinusitis phenotype and likely aetiology, in practice treatment may be initiated without knowledge of the polyp status, particularly in primary care. This review (and most of its companion reviews) consider patients with and without polyps together in the initial evaluation of treatment effects. However, subgroup analyses explore potential differences between them.
The most commonly used interventions for chronic rhinosinusitis are used either topically (sprayed into the nose) or systemically (by mouth) and include steroids, antibiotics and saline.
Description of the intervention
Various groups of systemic antibiotics have been studied in the treatment of chronic rhinosinusitis, including penicillins, cephalosporins, quinolones, tetracyclines and macrolides. The duration of antibiotic courses ranges from nine days to 12 weeks. Topical antibiotics have also been used to treat chronic rhinosinusitis. These have been delivered as antibiotic nasal washes and sprays.
How the intervention might work
Systemic and topical antibiotics are used in chronic rhinosinusitis with the aim of eliminating infection and inflammation, normalising the rheology and cohesivity of nasal mucus (Hatipoglu 2005; Inamura 2000; Miyanohara 2000; Wallwork 2006), altering bacterial biofilm formation (Wozniak 2004), reversing ostial occlusion and improving symptoms. The macrolide class of antibiotics has been specifically identified as potentially useful in chronic rhinosinusitis due to the well‐documented anti‐inflammatory effects of reducing cytokine activity and in turn reducing airway inflammation and mucus production (Tamaoki 2004), rather than for its antibacterial action. Topical antibiotics have the theoretical advantage of acting directly on the site of infection/inflammation and providing a higher concentration of antibiotic at the target site, but they have limited penetration into the sinuses in the un‐operated nose.
However, unnecessary antibiotic prescriptions should be avoided. Adverse effects are not uncommon, including allergy (MacLaughlin 2000); these are commonly manifested as skin irritation or rashes (and in severe cases as anaphylaxis, Stevens‐Johnson syndrome etc.), diarrhoea and abdominal pain (Bucher 2004). One of the main concerns with antibiotics is that overuse is associated with increasing resistance to antibiotics among community‐acquired pathogens.
Why it is important to do this review
Antibiotics are still frequently used to treat patients with chronic rhinosinusitis. This may be in the mistaken belief that in some patients with chronic rhinosinusitis some or all of their symptoms are related to the presence of pus in their sinuses or nasal secretions, or that 'sinus pain' is inevitably caused by 'sinus infection'. This review incorporates an update of a previous Cochrane review (Piromchai 2011), which evaluated systemic antibiotics but not topical ones. We sought to answer the important question of whether antibiotics are effective at all for patients with chronic rhinosinusitis, their relative effectiveness compared to other treatments and whether they are effective as an add‐on treatment. We also tried to find evidence to evaluate which types of antibiotic, dose or duration of treatment are effective.
This review is one of a suite of Cochrane reviews looking at common management options for patients with chronic rhinosinusitis (Chong 2016a; Chong 2016b; Chong 2016c; Head 2016a; Head 2016b), and we use the same outcome measures across the reviews. We have not included studies designed to evaluate interventions in the immediate peri‐surgical period, which are focused on assessing the impact of the intervention on the surgical procedure or on modifying the post‐surgical results (preventing recurrence of chronic rhinosinusitis symptoms).
Objectives
To assess the effects of systemic and topical antibiotics in people with chronic rhinosinusitis.
Methods
Criteria for considering studies for this review
Types of studies
We included studies with the following design characteristics:
randomised controlled trials, including cluster‐randomised trials and quasi‐randomised trials (cross‐over trials were only to be included if the data from the first phase were available); and
patients were followed up for at least two weeks.
We excluded studies with the following design characteristics:
randomised patients by side of nose (within‐patient controlled) because it is difficult to ensure that the effects of any of the interventions considered can be localised; or
perioperative studies, where the sole purpose of the study was to investigate the effect of the intervention on surgical outcome.
Types of participants
Patients with chronic rhinosinusitis, whether with polyps or without polyps.
We excluded studies that included a majority of patients with:
cystic fibrosis;
allergic fungal sinusitis/eosinophilic fungal/mucinous rhinosinusitis;
aspirin‐exacerbated respiratory disease;
antrochoanal polyps (benign polyps originating from the mucosa of the maxillary sinus);
malignant polyps;
primary ciliary dyskinesia;
a history of surgery for nasal polyps within six weeks of entry to the study;
allergic fungal rhinosinusitis/eosinophilic fungal/mucinous rhinosinusitis; or
aspirin‐exacerbated respiratory disease (aka Samter's triad).
Types of interventions
We included the following groups of antibiotics:
macrolides (e.g. clarithromycin, erythromycin);
tetracyclines (e.g. doxycycline);
beta‐lactams (e.g. penicillins/cephalosporins) with/without clavulanic acids;
quinolones.
We included both topically applied and oral antibiotics in the review. We included any dose and duration of treatment.
We defined short courses of antibiotics as up to 28 days, whereas we defined long‐term courses of antibiotics as longer than four weeks.
Comparisons
The comparators were:
placebo or no intervention;
another class of antibiotics;
-
the same type of antibiotic, which is either:
given for a different duration;
given at a different dose;
-
other treatments for chronic rhinosinusitis, including:
intranasal corticosteroids;
oral/systemic steroids;
the same type of antibiotic but given for a different duration;
the same type of antibiotic but given at a different dose.
Concurrent treatments were allowed if they were used in both treatment arms; they included:
nasal saline irrigation only;
intranasal corticosteroids only;
intranasal corticosteroids plus nasal irrigation;
intranasal corticosteroids plus nasal irrigation plus oral steroids;
intranasal corticosteroids plus oral steroids plus antifungal;
other combinations.
Comparison pairs
There were multiple possible comparison pairs due to the large number of interventions allowed.
The main comparison pairs of interest were:
antibiotics versus no intervention or placebo;
antibiotics plus intranasal steroids or other standard treatment versus no intervention or placebo plus intranasal steroids or other standard treatment.
Other possible comparison pairs included:
antibiotics versus intranasal steroids;
antibiotics versus oral/systemic steroids;
antibiotics class A versus antibiotics class B;
antibiotics plus oral steroids plus intranasal steroids versus oral plus intranasal steroids;
antibiotic A with duration of treatment X versus antibiotic A with duration of treatment Y;
antibiotic A at dose X versus antibiotic A at dose Y.
This review is part of a larger series of six reviews for the treatment of chronic rhinosinusitis.
Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis (Chong 2016b).
Different types of intranasal steroids for chronic rhinosinusitis (Chong 2016a). This review compares different classes, doses and delivery methods of intranasal corticosteroids for chronic rhinosinusitis.
Short‐course oral steroids alone for chronic rhinosinusitis (Head 2016a). This review compares short‐course oral steroids alone with placebo or no intervention, or against other pharmacological interventions such as antibiotics or nasal saline irrigation.
Short‐course oral steroids as an adjunct therapy for chronic rhinosinusitis (Head 2016b). This review compares oral steroids where they have been used as add‐on therapy to other treatments for chronic rhinosinusitis (such as intranasal corticosteroids, antibiotics or saline solution).
Saline irrigation for chronic rhinosinusitis (Chong 2016c). This review compares nasal saline irrigation for chronic rhinosinusitis with both placebo/no intervention and with intranasal corticosteroids or antibiotics.
Systemic and topical antibiotics for chronic rhinosinusitis (this review). This review compares both topical and systemic antibiotics with placebo/no treatment, two different antibiotics with each other and antibiotics with intranasal corticosteroids.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
Primary outcomes
Health‐related quality of life, using disease‐specific health‐related quality of life scores, such as the Sino‐Nasal Outcome Test‐22 (SNOT‐22), Rhinosinusitis Outcome Measures‐31 (RSOM‐31) and SNOT‐20.
Disease severity, as measured by patient‐reported symptom score (such as the Chronic Sinusitis Survey (CSS) questionnaire and visual analogue scales). In the absence of validated symptom score data, patient‐reported individual symptom scores were reported for the following symptoms: nasal obstruction/blockage/congestion, nasal discharge (rhinorrhoea), facial pressure/pain, loss of sense of smell (adults) and cough (children).
Significant adverse effect: gastrointestinal disturbances include nausea and vomiting, diarrhoea and abdominal pain.
Secondary outcomes
Health‐related quality of life, using generic quality of life scores, such as the SF‐36, EQ‐5D and other well‐validated instruments.
Other adverse effects: suspected allergic reaction (rash or skin irritation).
Other adverse effects: anaphylaxis or other very serious reactions (e.g. Stevens‐Johnson syndrome).
Endoscopic score (depending on population, either nasal polyps size score or endoscopy score, e.g. Lund‐Mackay/Lund‐Kennedy).
Computerised tomography (CT) scan score (e.g. Lund‐Mackay).
Both short‐term (at the end of treatment) and long‐term effects are important therefore we evaluated outcomes at the end of treatment or within three weeks, at three to six months, six to 12 months and more than 12 months. For adverse events, we analysed data from the longest time periods.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 29 September 2015.
Electronic searches
The Information Specialist searched:
the Cochrane Register of Studies ENT Trials Register (searched 29 September 2015);
the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 8);
-
Ovid MEDLINE (1946 to October week 1 2015);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 29 September 2015);
PubMed (as a top up to searches in Ovid MEDLINE) (searched 29 September 2015);
Ovid EMBASE (1974 to 2015 week 41);
ClinicalTrials.gov, www.clinicaltrials.gov (search via the Cochrane Register of Studies) (searched 29 September 2015);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (searched 29 September 2015);
Google Scholar (searched 29 September 2015).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched PubMed, The Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
Data collection and analysis
Selection of studies
At least two review authors independently screened all titles and abstracts of the studies obtained from the database searches to identify potentially relevant studies. At least two review authors evaluated the full text of each potentially relevant study to determine if it met the inclusion and exclusion criteria for this review.
We resolved any differences by discussion and consensus, with the involvement of a third author for clinical and/methodological input where necessary.
Data extraction and management
Two review authors independently extracted data from each study using a standardised data collection form (see Appendix 2). Whenever a study had more than one publication, we retrieved all publications to ensure complete extraction of data. Where there were discrepancies in the data extracted by different review authors, we checked these against the original reports and resolved differences by discussion and consensus, with the involvement of a third author or a methodologist where appropriate. We contacted the original study authors for clarification or for missing data whenever possible. If differences were found between publications of a study, we contacted the original authors for clarification. We used data from the main paper(s) if no further information was found.
We included key characteristics of the studies, such as study design, setting, sample size, population and how outcomes were defined or collected in the studies. In addition, we also collected baseline information on prognostic factors or effect modifiers. For this review, this included:
presence or absence of nasal polyps;
baseline nasal polyp score;
whether the patient has had previous sinus surgery.
We also noted down whether studies only selected patients with known bacterial colonisation.
For the outcomes of interest to the review, we extracted the findings of the studies on an available case analysis basis; i.e. we included data from all patients available at the time points based on the treatment randomised whenever possible, irrespective of compliance or whether patients had received the treatment as planned.
In addition to extracting pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias, we extracted the following summary statistics for each trial and each outcome:
For continuous data: the mean values, standard deviations and number of patients for each treatment group. Where endpoint data were not available, we extracted the values for change from baseline. We analysed data from measurement scales such as SNOT‐22 and EQ‐5D as continuous data.
For binary data: the numbers of participants experiencing an event and the number of patients assessed at the time point.
For ordinal scale data: if the data appeared to be approximately normally distributed or if the analysis that the investigators performed suggested parametric tests were appropriate, then we treated the outcome measures as continuous data. Alternatively, if data were available, we planned to convert into binary data.
We prespecified the time points of interest for the outcomes in this review. While studies may have reported data at multiple time points, we only extracted the longest available data within the time points of interest. For example, for 'short' follow‐up periods, our time point was defined as 'three to six months' post‐randomisation. If a study had reported data at three, four and six months, we only extracted and analysed the data for the six‐month follow‐up.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each included study. We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), and we used the Cochrane 'Risk of bias' tool. With this tool we assessed the risk of bias as 'low', 'high' or 'unclear' for each of the following six domains:
sequence generation;
allocation concealment;
blinding of participants, personnel and outcome assessment;
incomplete outcome data;
selective reporting;
other sources of bias.
Measures of treatment effect
We summarised the effects of dichotomous outcomes (e.g. proportion of patients with symptom resolution) as risk ratios (RR) with CIs. For the key outcomes that we presented in the 'Summary of findings' table, we also expressed the results as absolute numbers based on the pooled results and compared to the assumed risk. We also planned to calculate the number needed to treat to benefit (NNTB) using the pooled results. The assumed baseline risk is typically either (a) the median of the risks of the control groups in the included studies, this being used to represent a 'medium risk population' or, alternatively, (b) the average risk of the control groups in the included studies is used as the 'study population' (Handbook 2011). If a large number of studies had been available, and where appropriate, we had also planned to present additional data based on the assumed baseline risk in (c) a low‐risk population and (d) a high‐risk population.
For continuous outcomes, we expressed treatment effects as a mean difference (MD) with standard deviation (SD) (we would have used the standardised mean difference (SMD) if different scales had been used to measure the same outcome, and we would have provided a clinical interpretation of the SMD values).
Unit of analysis issues
This review did not use data from phase II of cross‐over studies or from studies where the patient was not the unit of randomisation, i.e. studies where the side (right versus left) was randomised.
If we had found cluster‐randomised trials, we would have analysed these according to the methods in section 16.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Dealing with missing data
We tried to contact study authors via email whenever the outcome of interest was not reported, if the methods of the study suggested that the outcome had been measured. We did the same if not all data required for meta‐analysis were reported, unless the missing data were standard deviations. If standard deviation data were not available, we approximated these using the standard estimation methods from P values, standard errors or 95% CIs if these were reported, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). Where it was impossible to estimate these, we contacted the study authors.
Apart from imputations for missing standard deviations, we conducted no other imputations. However, we completed calculations relating to disease severity (measured by patient‐reported symptom scores) as most of the data measured individual symptoms rather than using validated instruments (see 'Imputing total symptom scores' below). We extracted and analysed data for all outcomes using the available case analysis method.
Imputing total symptom scores
Where a paper did not present information for the total disease severity in terms of patient‐reported symptom scores but did present data for the results of individual symptoms, we used the symptoms covering the important domains of the EPOS chronic rhinosinusitis diagnosis criteria (EPOS 2012), to calculate a total symptom score. The EPOS 2012 criteria for chronic rhinosinusitis require at least two symptoms. One of the symptoms must be either nasal blockage or nasal discharge; other symptoms can include facial pressure/pain, loss of sense of smell (for adults) or cough (for children). Where mean final values or changes from baseline were presented in the paper for the individual symptoms we summed these to calculate a 'total symptom score'. We calculated standard deviations for the total symptom score as if the symptoms were independent, random variables that were normally distributed. We acknowledge that there is likely to be a degree of correlation between the individual symptoms, however we used this process because the magnitude of correlation between the individual symptoms is not currently well understood (no evidence found). If the correlation is high, the summation of variables as discrete variables is likely to give a conservative estimate of the total variance of the summed final score. If the correlation is low, this method of calculation will underestimate the standard deviation of the total score. However, the average patient‐reported symptom scores have a correlation coefficient of about 0.5; if this is also applicable to chronic rhinosinusitis symptoms, the method used should have minimal impact (Balk 2012). As this method of calculation does not take into account weighting of different symptoms (no evidence found), we downgraded all the disease severity outcomes for lack of use of validated scales whenever this occurred.
Assessment of heterogeneity
We assessed clinical heterogeneity (which may be present even in the absence of statistical heterogeneity) by examining the included trials for potential differences between studies in the types of participants recruited, interventions or controls used and the outcomes measured.
We would have assessed statistical heterogeneity by visually inspecting the forest plots and by considering the Chi² test (with a significance level set at P value < 0.10) and the I² statistic, which calculates the percentage of variability that is due to heterogeneity rather than chance, with I² values over 50% suggesting substantial heterogeneity (Handbook 2011).
Assessment of reporting biases
We assessed reporting bias as between‐study publication bias and within‐study outcome reporting bias.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report against the study protocol, whenever this could be obtained. If the protocol was not available, we compared the outcomes reported to those listed in the methods section. If results were mentioned but not reported adequately in a way that allowed analysis (e.g. the report only mentioned whether the results were statistically significant or not), bias in a meta‐analysis is likely to occur. We sought further information from the study authors. If no further information could be obtained, we noted this as being a 'high' risk of bias. Quite often there was insufficient information to judge the risk of bias; we noted this as an 'unclear' risk of bias (Handbook 2011).
Publication bias (between‐study reporting bias)
We planned to assess funnel plots if sufficient trials (more than 10) had been available for an outcome. If we had observed asymmetry of the funnel plot, we had planned to conduct more formal investigation using the methods proposed by Egger 1997.
Data synthesis
We conducted all meta‐analyses using Review Manager 5.3 (RevMan 2014). For dichotomous data, we planned to analyse treatment differences as a risk ratio (RR) calculated using the Mantel‐Haenszel methods. We planned to analyse time‐to‐event data using the generic inverse variance method.
For continuous outcomes, if all the data were from the same scale, we had planned to pool mean values obtained at follow‐up with change outcomes and report this as a MD. However, if the SMD had to be used as an effect measure, we would not have pooled change and endpoint data.
When statistical heterogeneity is low, random‐effects versus fixed‐effect methods yield trivial differences in treatment effects. However, when statistical heterogeneity is high, the random‐effects method provides a more conservative estimate of the difference.
Subgroup analysis and investigation of heterogeneity
We had planned to conduct some subgroup analyses regardless of whether statistical heterogeneity was observed, as these are widely suspected to be potential effect modifiers. For this review, this included:
Phenotype of patients: whether patients have chronic rhinosinusitis without nasal polyps, chronic rhinosinusitis with nasal polyps, a mixed group or the status of polyps is not known or not reported. We planned to undertake the subgroup analysis as although there appears to be a considerable overlap between the two forms of chronic rhinosinusitis with regards to inflammatory profile, clinical presentation and effect of treatment (Cho 2012; DeMarcantonio 2011; Ebbens 2010; Fokkens 2007; Ragab 2004; Ragab 2010; van Drunen 2009), there is some evidence pointing to differences in the respective inflammatory profiles (Kern 2008; Keswani 2012; Tan 2011; Tomassen 2011; Zhang 2008; Zhang 2009), and potentially even differences in treatment outcome (Ebbens 2011). The role of microbes in the pathology is also unclear and this makes it uncertain whether antibiotics will have similar effects.
Class of antibiotics: some antibiotics, such as the macrolides, are known to have some anti‐inflammatory actions in addition to their antibacterial activity.
We planned to present the main analyses of this review according to the subgroups of phenotypes of chronic rhinosinusitis. We intended to present all other subgroup analysis results in tables.
When studies had a mixed group of patients, we planned to analyse the study as one of the subgroups (rather than as a mixed group) if more than 80% of patients belonged to one category. For example, if 81% of patients had chronic rhinosinusitis without nasal polyps, we would have analysed the study as that subgroup.
In addition to the subgroups above, we planned to conduct the following subgroup analyses in the presence of statistical heterogeneity:
patient age (children versus adults);
dose;
duration of treatment;
method of delivery (dependent on review).
Sensitivity analysis
We planned to carry out sensitivity analyses to determine whether the findings were robust to the decisions made in the course of identifying, screening and analysing the trials. We planned to conduct sensitivity analysis for the following factors, whenever possible:
impact of model chosen: fixed‐effect versus random‐effects model;
risk of bias of included studies: excluding studies with high risk of bias (we defined these as studies that have a high risk of allocation concealment bias and a high risk of attrition bias (overall loss to follow‐up of 20%, differential follow‐up observed);
how outcomes were measured: we planned to investigate the impact of including data where the validity of the measurement was unclear.
If any of these investigations had found a difference in the size of the effect or heterogeneity, we would have mentioned this in the Effects of interventions section.
GRADE and 'Summary of findings' table
We used the GRADE approach to rate the overall quality of evidence using the GDT tool (http://www.guidelinedevelopment.org/) for the main comparison pairs listed in the Types of interventions section. The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct and we applied this in the interpretation of results. There are four possible ratings: 'high', 'moderate', 'low' and 'very low'. A rating of 'high' quality evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of 'very low' quality implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high quality. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision;
publication bias.
The 'Summary of findings' tables present only the seven top priority outcomes (disease‐specific health‐related quality of life, disease severity score, adverse effects and generic quality of life score). We did not include the outcomes endoscopic score and CT scan score in the 'Summary of findings' tables.
Results
Description of studies
Results of the search
The searches retrieved a total of 2761 references (after removal of duplicates). We screened the titles and abstracts and subsequently removed 2698 studies. We assessed 63 full texts for eligibility. We included five studies (six papers) and excluded 47 studies (52 papers). Five studies are awaiting assessment. We identified one ongoing study.
A flow chart of study retrieval and selection is provided in Figure 1.
1.
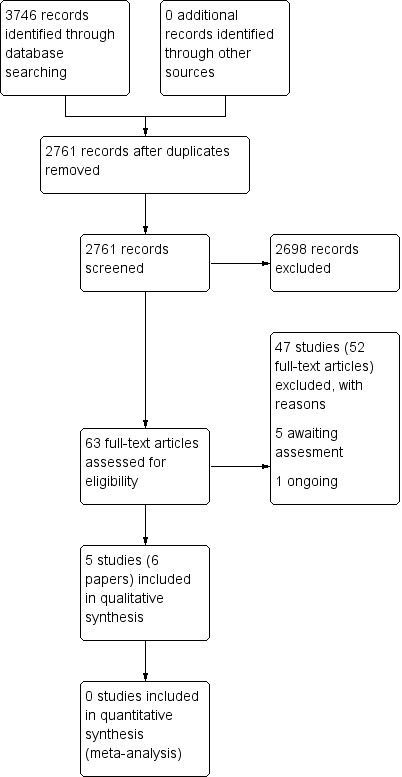
Process for sifting search results and selecting studies for inclusion.
Included studies
There are five studies (six papers) with 293 participants included in this review (Otten 1994; Van Zele 2010; Videler 2011; Wallwork 2006; Zeng 2011). The important characteristics of these studies are summarised below.
Design
Four studies were double‐blind, parallel‐group RCTs (Otten 1994; Van Zele 2010; Videler 2011; Wallwork 2006). One study was an open‐label, parallel‐group RCT (Zeng 2011). All of the included studies had a two‐arm design except Van Zele 2010, which used a three‐arm design. All studies had a minimum of 12 weeks follow‐up.
Setting
One study was a single‐centre trial conducted in China (Zeng 2011). Two studies were multicentre trials conducted in a single country: the Netherlands (Otten 1994) and Australia (Wallwork 2006). The remaining two were international, multicentre trials: Van Zele 2010 took place in five centres in Belgium, Germany, Holland and Australia and Videler 2011 was conducted in six centres in the Netherlands, Finland, Belgium, England and Croatia.
Participants and sample sizes
The sample sizes ranged from 43 (Zeng 2011) to 79 participants (Otten 1994). Four studies included an adult population ranging in age from 20 to 70 years (Van Zele 2010; Videler 2011; Wallwork 2006; Zeng 2011), and the last recruited only a paediatric population with an age range of 2 to 12 years (Otten 1994).
The European Position Paper on Rhinosinusitis and Nasal Polyps (EP3OS) diagnostic criteria (EPOS 2007) were used in two studies (Videler 2011; Zeng 2011). Zeng 2011 only recruited Chinese people with chronic rhinosinusitis without nasal polyps. Videler 2011 recruited people with recalcitrant chronic rhinosinusitis (absence of response to standard treatment) both with and without nasal polyps, although people with nasal polyps assessed as being equal to 2 or more on a 0 to 3 scale (0 = no polyps, 3 = severe polyps) were excluded. Nasal polyps were present in 62.1% and 41.9% of participants in the antibiotics and placebo groups, respectively. Wallwork 2006 recruited patients based on the Rhinosinusitis Task Force (Lanza 1997); nasal polyps were an exclusion criterion.
One study recruited participants based on the endoscopic finding of recurrent bilateral nasal polyps after surgery or massive bilateral nasal polyps (grade 3 or 4 on a scale of 0 to 4) (Van Zele 2010).
Otten 1994 included a paediatric population with chronic sinusitis diagnosed using a combination of clinical signs and symptoms for more than three months and radiographic findings. Nasal polyps were an exclusion criterion. The age range was from 2 to 12 years.
All studies had almost equal numbers of male and female participants (Otten 1994; Van Zele 2010; Videler 2011; Wallwork 2006; Zeng 2011).
Interventions
Four studies were placebo‐controlled (Otten 1994; Van Zele 2010; Videler 2011; Wallwork 2006). One study compared antibiotics to oral corticosteroids (Van Zele 2010), and one to intranasal corticosteroids (Zeng 2011).
The antibiotics under investigation were cefaclor (Otten 1994), roxithromycin (Wallwork 2006), azithromycin (Videler 2011), clarithromycin (Zeng 2011), and doxycycline (Van Zele 2010).
Otten 1994 compared cefaclor at a dose of 20 mg/kg/day divided into three equal doses for one week with placebo. Wallwork 2006 compared roxithromycin 150 mg daily for three months with placebo. Videler 2011 compared oral azithromycin 500 mg per day for three days, then 500 mg per week for 11 weeks with placebo. Zeng 2011 compared oral clarithromycin 250 mg once daily for 12 weeks with mometasone furoate nasal spray 200 µg once daily for 12 weeks. Van Zele 2010 had two separate comparisons (doxycycline versus placebo and doxycycline versus oral methylprednisolone). Doxycycline was given at 200 mg on day 1, 100 mg/day on days 2 to 20 and oral methylprednisolone was given at 32 mg/day on days 1 to 5, at 16 mg/day on days 6 to 10 and at 8 mg/day on days 11 to 20.
Three studies mention that participants were not allowed to use some treatments in the four weeks prior to the trial. Wallwork 2006 excluded people who had used intranasal steroids or oral steroids, Videler 2011 excluded people who had used oral steroids, and Zeng 2011 excluded people who had used saline irrigation, intranasal steroids, oral steroids or macrolide antibiotic treatment.
With regard to concomitant treatments during the trial, Zeng 2011 commented that the patients did not receive other additional treatments during the trial and two studies made no mention of other treatments (Otten 1994; Wallwork 2006). Both study groups in Videler 2011 received nasal saline irrigation and Otten 1994 comments that saline was used to aspirate pus for cultures. Where no mention of additional treatment was made it is possible that the participants performed saline irrigations as it was neither specifically included nor excluded (Otten 1994; Van Zele 2010; Wallwork 2006).
Two trials provided information on concomitant intranasal steroid treatment: in Van Zele 2010 the use of concomitant intranasal steroids during the treatment phase was not allowed, although the authors state that it was allowed as rescue medication in the follow‐up period once the treatment phase had been completed. The second trial, Videler 2011, allowed concurrent intranasal steroids providing the dose was kept constant throughout study and reported that 70% of the patients received intranasal steroids.
Outcomes
Disease‐specific health‐related quality of life
This was measured in two studies (Videler 2011; Wallwork 2006). Videler 2011 used the Sinonasal Outcome Test‐22 (SNOT‐22) score (range: 0 to 110), measured at baseline, 14 and 24 weeks, whereas Wallwork 2006 used the Sinonasal Outcome Test‐20 (SNOT‐20) (range: 0 to 5) at pre‐treatment, at 12 weeks immediately after treatment and at 24 weeks (12 weeks post treatment).
Disease severity ‐ symptoms score
Three studies presented information about disease severity in terms of symptom scores (Van Zele 2010; Videler 2011; Zeng 2011). Van Zele 2010 used a disease severity score, measured by patient‐assessed symptoms (anterior rhinorrhoea, nasal obstruction, post‐nasal drip and loss of sense of smell) at 20 days and 12 weeks although details of the scales used to record symptoms are not provided within the paper.
Videler 2011 evaluated the symptoms of headache, nasal obstruction, rhinorrhoea, post‐nasal drip, feeling of fullness, smell disturbance, facial pain, toothache, tears, coughing, nasal bleeding and crusts on a 0 to 10 visual analogue scale (VAS) at 12 weeks. The study also reported an overall change in symptoms using "Patient Response Rating Scale" to classify the subjective effect of the course (‐2 desperately worse (deterioration of symptoms with significant impact on normal life); ‐1 worse (compared with the pretreatment situation); 0 no change; 1 improvement (although symptoms are present, they are scarcely troublesome); and 2 cured (virtually no symptoms present)).
Zeng 2011 scored five symptoms (nasal obstruction, rhinorrhoea, loss of sense of smell, facial pain or pressure, headache) using a 0 to 10 VAS at 0, 4, 8 and 12 weeks.
General health‐related quality of life
This was measured in one study, Videler 2011, which used the Short Form‐36 (SF‐36) instrument at baseline, 14 and 24 weeks.
Endoscopic scores (including nasal polyps score)
Four studies recorded endoscopic scores (Van Zele 2010; Videler 2011; Wallwork 2006; Zeng 2011). Three of these looked at overall endoscopic findings (Videler 2011; Wallwork 2006; Zeng 2011). One study used the validated Lund‐Kennedy score measuring swelling and discharge (Zeng 2011). The other studies did not provide information about validation of the scales used (Videler 2011; Wallwork 2006). One study only assessed the size of the nasal polyps (Van Zele 2010). The measurements were made pre‐treatment and after treatment (12 weeks) in all studies (Van Zele 2010; Videler 2011; Wallwork 2006; Zeng 2011).
Computerised tomography (CT) scan score
No studies provided results for this outcome.
Adverse effects
Two studies reported information on adverse events (Van Zele 2010; Videler 2011). Van Zele 2010 recorded gastrointestinal disturbances (reflux or gastric pain, or both), and skin irritation (skin rash). No studies reported anaphylaxis or other serious allergic reactions such as Stevens‐Johnson syndrome. Videler 2011 reported that there were no serious adverse effects and reported gastrointestinal disturbances (mostly mild diarrhoea). No other studies made any comment about adverse events.
Funding and conflict of interests in trials
No information about funding for the trial was provided in two studies (Otten 1994; Wallwork 2006). In one the study medication was provided by the pharmaceutical company but no further information was provided (Videler 2011). The remaining two studies were funded by what appear to be academic or governmental grants (Van Zele 2010; Zeng 2011).
Two studies did not provide information on any potential conflicts of interest of investigators within the trials (Otten 1994; Wallwork 2006). Two stated that they knew of no known conflicts of interest (Videler 2011; Zeng 2011). One reported that one author had received royalties from a medical device company and was a consultant for another company (NeilMed). This author, along with two other authors, received research grants from external bodies (Garnett Passe and Rodney Williams Foundation, GlaxoSmithKline, Stallergenes, European Union) (Van Zele 2010).
Excluded studies
We excluded 47 studies (52 papers) after reviewing the full paper. Further details of the reasons for exclusion are summarised in Characteristics of excluded studies.
We excluded most of the studies (32) due to the duration of follow‐up in the trial not meeting the minimum criteria (three months) as set out in the review protocol inclusion criteria (Agbim 1975; Amini 2009Ansari 2015; Artigas 1989; Beloborodova 1998; Bonfils 2015; Dellamonica 1994; Desrosiers 2001; Edelstein 1993; El'kun 1999; Fan 2014; Huck 1993; Husfeldt 1993; Jareoncharsri 2004; Jervis‐Bardy 2012; Jiang 2008; Korkmaz 2014; Kunel'skaya 2008; Legent 1994; Li 2000; Li 2002; Li 2014; Mannhardt 1980; Namyslowski 1998; Peric 2011; Portier 1996; Rachelefsky 1982; Sreenath 2015; Sykes 1986; Videler 2008; Watanabe 2003; Wei 2011). Of these studies, 18 followed up participants for one month or less (Agbim 1975; Ansari 2015; Artigas 1989; Beloborodova 1998; Edelstein 1993; El'kun 1999; Huck 1993; Husfeldt 1993; Jareoncharsri 2004; Jervis‐Bardy 2012; Jiang 2008; Kunel'skaya 2008; Li 2014; Mannhardt 1980; Portier 1996; Rachelefsky 1982; Sykes 1986; Watanabe 2003). These studies generally included a mixed population of acute sinusitis and participants with acute exacerbations of chronic sinusitis. Participants were randomised to a short course of oral steroids (7 to 14 days) and followed up at the end of the treatment period or up to two weeks after treatment. Seven studies followed up patients for four to eight weeks (Bonfils 2015; Dellamonica 1994; Fan 2014; Legent 1994; Namyslowski 1998; Sreenath 2015; Wei 2011), and four studies for between eight to 12 weeks (Amini 2009; Desrosiers 2001; Korkmaz 2014; Peric 2011). There were three studies where the duration of follow‐up was not clear (Li 2000; Li 2002; Videler 2008); this included one study that was a cross‐over trial (Videler 2008). In this study the outcomes were reported after the first phase at between 8 to 12 weeks, but the precise timing was not clear. In addition, the results of the first and second phase were reported together and it was not possible to separate these out. We attempted to contact the study author for further information but could not establish contact.
We excluded nine studies because the study aims were to investigate the effectiveness of antibiotics in the perioperative, or immediate postoperative, period (Amali 2015; Bobacheva 2012; Chatzimanolis 1998; Haxel 2015; Hiratsuka 1996; NCT01825408; NCT02307825; Schalek 2009; Varvianskaia 2013).
Three studies made comparisons that were not relevant to this review (Hashiba 1997; IRCT201312299014N; Otten 1997). Hashiba 1997 compared clarithromycin with erythromycin, which were both the same class of antibiotic and, therefore, excluded from the review. IRCT201312299014N is an ongoing trial that will compare phonophoresis of erythromycin with pulsed ultrasound. Otten 1997 compared four different interventions: xylometazoline (nasal decongestant) plus antibiotics and drainage, drainage plus xylometazoline plus antibiotics, and a placebo arm.
We excluded two studies due to the design of the study (Bezerra 2014; Kita 1995): both were non‐randomised controlled trials.
We excluded one study as the included population were those with sinobronchial syndrome (Ishiura 1995).
Studies awaiting assessment
There are five studies awaiting assessment. For four studies the full paper could not be obtained within the timeframe of the review (Behm 2002; Jiang 2001; Kataoka 2003; Ziuzio 1995), and for the fifth the length of follow‐up in the study was unclear from the paper (Kim 2003). We have contacted the study author and we are awaiting a reply. See Characteristics of studies awaiting classification.
Ongoing studies
We identified one ongoing study (EUCTR 2005 (2005‐004736‐51)). See Characteristics of ongoing studies.
Risk of bias in included studies
The included studies were all randomised and controlled. Details of the risk of bias for each study can be found in Figure 2. A 'Risk of bias' graph shows our judgements about each risk of bias item presented as percentages across all included studies (Figure 3). In general the reporting of the trials was not of a high quality.
2.
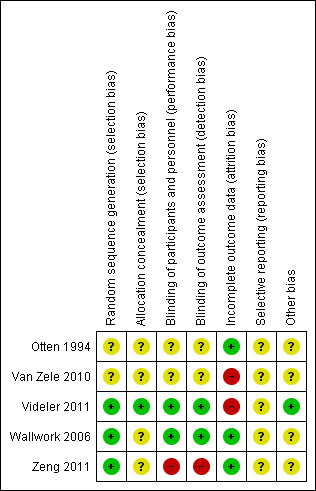
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
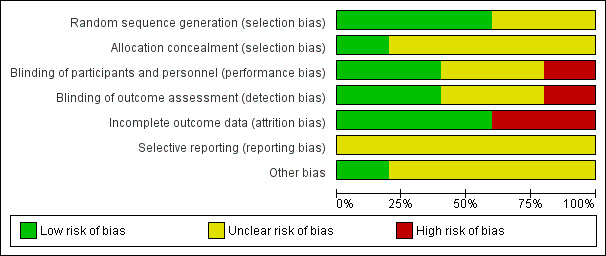
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
We assessed three studies as having a low risk of bias for sequence generation as the methods were well described (Videler 2011; Wallwork 2006; Zeng 2011). Two studies did not provide any information about how patients were randomised (Otten 1994; Van Zele 2010). In Van Zele 2010, the number of people randomised was small and there is a risk that the allocation between the groups many not have been balanced (14 in group 1, 14 in group 2 and 19 in group 3).
Allocation concealment
Four studies did not present any information about the methods for allocation concealment (Otten 1994; Van Zele 2010; Wallwork 2006; Zeng 2011). We assessed Videler 2011 to be at low risk of bias for allocation concealment as the randomisation occurred externally and randomised packs were distributed to the participants who qualified with consecutive numbering.
Baseline characteristics
In two studies there was a lack of information on baseline characteristics of the study participants (Otten 1994; Wallwork 2006), although Wallwork 2006 did report that there were no differences between the two groups. Van Zele 2010 reported an imbalance between the two groups in the number of participants at baseline with 'allergy' (oral steroids: 35.7%; placebo: 57.9%; antibiotics: 14.3%) and the number who were "aspirin intolerant" (oral steroids: 14.3%; placebo: 26.3%; antibiotics: 7.1%). These were not statistically different but it may have been due the sample size being small (n = 48). The other studies did not find differences in the baseline characteristics between their study arms (Videler 2011; Zeng 2011).
Blinding
We assessed two studies to be at a low risk of performance and detection bias as they were both double‐blind studies with both patients and healthcare professionals (outcome assessors) blinded to the treatment group (Videler 2011; Wallwork 2006).
We assessed two studies as having an unclear risk of bias due to blinding (Otten 1994; Van Zele 2010). Both studies stated that they were "double‐blind" but did not provide any further information. Van Zele 2010 provides no information about the dosing schedule of the three arms within the trial (oral steroids, placebo and antibiotics) and what precautions were taken to prevent the participants and healthcare professionals from identifying the treatment arm to which they had been allocated. There was no information about blinding of outcome assessment in the paper.
Zeng 2011 was an open‐label study and so we assessed the risk of bias due to blinding as high.
Incomplete outcome data
Three studies were at a low risk of attrition bias with no patients reported as dropping out of the study in Zeng 2011, a drop‐out rate (with reasons provided) of 5% in Otten 1994, and 8% at the end of treatment and 12.5% at the follow‐up six months from the start of treatment in Wallwork 2006. There were no significant differences in drop‐out rates between the groups in any study.
We assessed both Van Zele 2010 and Videler 2011 to be at a high risk of bias due to incomplete outcome data. In Van Zele 2010, seven of the initial 47 patients dropped out of the study (14.9%) and an intention‐to‐treat analysis was conducted with the last value carried forward. However, all of the patients who dropped out were from the placebo group 7/19 (36.8%). The report implies that they all dropped out after the treatment stage during follow‐up. This may have had an effect on the overall results and no sensitivity analysis appears to have been completed to identify the impact. In Videler 2011, 9/60 participants (15%) did not complete the study. Reasons for non‐completion are given and are distributed equally between the intervention and placebo group. No denominator values were given by group at the final time point, although additional participants dropping out are reported. Denominator values for measuring adverse events (gastrointestinal disturbances) are not reported.
Selective reporting
We assessed all of the studies to be at an unclear risk of bias due to selective reporting (Otten 1994; Van Zele 2010; Videler 2011; Wallwork 2006; Zeng 2011). In Van Zele 2010, many of the results were presented graphically, without providing values at key time periods. The data were not reported in a way that allowed them to be included in the meta‐analysis for this review. Similarly, Videler 2011 did not always present the full results for all outcomes and these were sometimes reported generally rather than by providing the data (e.g. endoscopic score). In Otten 1994, the results are not well presented.
The reporting of adverse events was a particular concern in all of the studies (Otten 1994; Van Zele 2010; Videler 2011; Wallwork 2006; Zeng 2011). No information about whether there were any adverse events was given in three studies (Otten 1994; Wallwork 2006; Zeng 2011). Even in those studies that did present adverse events the methods for recording their measurement were not described (Van Zele 2010; Videler 2011).
A protocol document could be found for two of the five studies (Van Zele 2010; Videler 2011). For Van Zele 2010 (NCT00480298), it was difficult to judge whether there were differences between the protocol and the full paper as the protocol was not detailed. In Videler 2011 (EUCTR‐2005‐001062‐14), there is an additional outcome that is reported in the paper but not presented in the protocol: patient response scale, which was not one of the key outcomes in this review. In both cases we noted that the number of participants that the study aimed to recruit was different from the number actually recruited: 120 planned and 48 recruited for Van Zele 2010, and 120 planned and 60 recruited for Videler 2011.
Other potential sources of bias
Use of validated outcome measures
Although the studies reporting health‐related quality of life outcomes used validated instruments (Videler 2011; Wallwork 2006), the papers generally lacked information on the validation of other instruments. In particular, the validation of instruments used to measure 'symptom severity' was poorly reported. None of the three studies that reported this outcome mentioned validation (Van Zele 2010; Videler 2011; Zeng 2011). In fact, details of the scales used to measure symptoms were not provided in Van Zele 2010. It was a similar story with regard to the use of validated outcome measures for the endoscopic outcomes; three studies did not provide information on validation (Van Zele 2010; Videler 2011; Wallwork 2006).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Systemic antibiotics compared with placebo.
| Systemic antibiotics compared with placebo for chronic rhinosinusitis | ||||||
|
Patient or population: chronic rhinosinusitis Intervention: systemic antibiotics Comparison: placebo | ||||||
|
Outcomes № of participants (studies) |
Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without systemic antibiotics | With systemic antibiotics | Difference | ||||
| Disease‐specific HRQL
assessed with: SNOT‐20
Scale from: 0 to 5
Follow‐up: after treatment (3 months) and 3 months after treatment has ended (6 months) № of participants: 64 (1 RCT) |
— |
|
|
|
⊕⊕⊕⊝ MODERATE 1 |
|
| Disease severity ‐ patient‐reported symptoms | None of the studies reported this as an outcome | |||||
| Gastrointestinal disturbances
Follow‐up: 3 months № of participants: 33 (1 RCT) |
RR 1.36 (0.22 to 8.50) | Study population | ⊕⊝⊝⊝ VERY LOW 2 | It is uncertain whether there is an increase in gastrointestinal disturbances with antibiotics. | ||
| 105 per 1000 |
143 per 1000 (23 to 895) |
38 more per 1000 (82 fewer to 789 more) |
||||
| General health‐related quality of life | None of the studies reported this as an outcome | |||||
| Suspected allergic reaction (rash or skin irritation)
Follow‐up: 3 months № of participants: 33 (1 RCT) |
RR 6.67 (0.34 to 128.86) | Study population | ⊕⊝⊝⊝ VERY LOW 2 | It is uncertain whether there is an increase in skin irritation with antibiotics. No events were reported in the control arm. |
||
| 0 per 1000 | ||||||
| Anaphylaxis or other very serious reactions (e.g. Stevens‐Johnson syndrome)
Follow‐up: 3 months № of participants: 33 (1 RCT) |
No events were reported in either arm. The effect size could not be estimated. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;HRQL: health‐related quality of life; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference; SNOT‐20: Sino‐Nasal Outcome Test‐20 | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded to moderate quality due to imprecision: small sample size (n = 64) leading to imprecise results.
2Downgraded to low quality due to limitations of study design (lack of information about randomisation, allocation concealment and blinding, high risk of reporting bias) and imprecision (small study (n = 33) with low number of events leading to large confidence intervals).
3Downgraded to very low quality due to limitations of study design (lack of information about randomisation, allocation concealment and blinding, high risk of reporting bias), indirectness of the included population and intervention (study included a population who were more severely affected; those with recurrent polyps or recalcitrant disease; and the intervention was a 20‐day course of antibiotics) and imprecision (small study (n = 33) with no events in either arm).
Summary of findings 2. Systemic antibiotics plus saline irrigation and intranasal corticosteroids compared with placebo plus saline irrigation and intranasal corticosteroids.
| Systemic antibiotics plus saline irrigation and intranasal corticosteroids compared with placebo plus saline irrigation and intranasal corticosteroids for chronic rhinosinusitis | ||||||
|
Patient or population: participants with chronic rhinosinusitis Intervention: systemic antibiotics (macrolide) plus saline irrigation and intranasal corticosteroids Comparison: placebo plus saline irrigation and intranasal corticosteroids | ||||||
|
Outcomes № of participants (studies) |
Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without systemic antibiotics3 | With systemic antibiotics3 | Difference | ||||
| Disease‐specific HRQL assessed with: SNOT‐22 Follow‐up: 14 weeks and 6 months № of participants: 53 (1 RCT) |
The data were highly skewed. Median values were not presented in the paper. The paper reports no statistically significant differences between the groups at any of the time points (P values < 0.05 using the Mann Whitney U‐test). | ⊕⊕⊝⊝ LOW1 | It is unclear whether there is an important difference between groups. The data are difficult to interpret because: 1) the baseline score was higher in the antibiotics groups, i.e. worse (antibiotics = 48.2, placebo = 40.0); 2) the data were highly skewed. Only the mean and SD values were reported, and these are not good estimates of the average change in patients. |
|||
| Disease severity assessed with: 5‐point "patient response" scale and dichotomised into people who improved versus people who remain unchanged or had worsened symptoms Follow‐up: 3 months No of participants: 56 (1 RCT) |
RR 1.50 (0.81 to 2.79) |
345 per 1000 | 517 per 1000 (279 to 962) |
172 more per 1000 (66 fewer to 617 more) |
⊕⊝⊝⊝ VERY LOW2 | The proportion of patients who improved was potentially slightly higher in the antibiotics group. However, there were also patients who worsened. |
| Gastrointestinal disturbances Follow‐up: 3 months № of participants: 56 (1 RCT) |
RR 1.07 (0.16 to 7.10) |
Study population | ⊕⊕⊝⊝ LOW1 | It is very uncertain whether there is a difference between the groups. | ||
| 69 per 1000 |
74 per 1000 (11 to 490) |
5 more per 1000 (58 fewer to 421 more) |
||||
| General health‐related quality of life assessed with: SF‐36 Follow‐up: 3 months № of participants: 56 (1 RCT) |
One study reported the SF‐36 at the end of treatment (12 weeks) (n = 56) and 2 weeks after the end of treatment (14 weeks) (n = 53). The study authors noted that there were no statistically significant differences for any of the SF‐36 domains between the group receiving antibiotic plus saline irrigation and intranasal corticosteroids and the group receiving placebo plus saline irrigation and intranasal corticosteroids. No further information is presented. | |||||
| Suspected allergic reaction (rash or skin irritation) | The study did not report this as an outcome. | |||||
| Anaphylaxis or other very serious reactions (e.g. Stevens‐Johnson syndrome) Follow‐up: 3 months № of participants: 33 (1 RCT) |
No events were reported in either arm. The effect size could not be estimated. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;HRQL: health‐related quality of life; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference; SNOT‐22: Sino‐Nasal Outcome Test‐22 | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded to low quality: participants included in the trial had more severe, recalcitrant CRS compared with the average CRS population. The trial is very small (n = 53) and the results are imprecise.
2Downgraded to very low quality: participants included in the trial had more severe, recalcitrant CRS compared with the average CRS population. The trial is very small (n = 53), the results are imprecise and there is no information provided about the validation of the rating scale.
3All patients in both study arms received nasal saline irrigation and most patients (70%) received intranasal corticosteroids.
Summary of findings 3. Systemic antibiotics compared with intranasal corticosteroids.
| Systemic antibiotics compared with intranasal corticosteroids for chronic rhinosinusitis | ||||||
|
Patient or population: chronic rhinosinusitis Intervention: systemic antibiotics (macrolide) Comparison: intranasal corticosteroids | ||||||
|
Outcomes № of participants (studies) |
Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without systemic antibiotics | With systemic antibiotics | Difference | ||||
| Disease‐specific HRQL | The study did not report this as an outcome | |||||
| Disease severity, as measured by patient‐reported symptom score (0 to 40),
measured by combining 4 individual symptoms2 Follow‐up: 3 months № of participants: 43 (1 RCT) |
— | The mean disease severity score without systemic antibiotics was 6 | The mean disease severity score in the intervention group was 0.32 lower (2.11 lower to 1.47 higher) | MD 0.32 lower (2.11 lower to 1.47 higher) |
⊕⊕⊝⊝ LOW1 | Lower scores indicate less severe symptoms (possible range 0 to 40) It is very uncertain whether there was a difference in disease severity (as measured by combined symptoms score) between the groups. The mean difference corresponds to a small effect size (SMD 0.11). |
| Gastrointestinal disturbances | The study did not provide any information about adverse events | |||||
| General health‐related quality of life | The study did not report this as an outcome | |||||
| Suspected allergic reaction (rash or skin irritation) | The study did not provide any information about adverse events | |||||
| Anaphylaxis or other very serious reactions (e.g. Stevens‐Johnson syndrome) | The study did not provide any information about adverse events | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;HRQL: health‐related quality of life; MD: mean difference; RCT: randomised controlled trial; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded to low quality due to risk of bias due to study design (open‐label study, lack of information about allocation concealment) and small number of participants (n = 43) leading to imprecise results.
2Symptoms included in overall symptom score were: nasal obstruction, rhinorrhoea, loss of sense of smell and facial pain.
Summary of findings 4. Systemic antibiotics compared with oral corticosteroids.
| Systemic antibiotics compared with oral corticosteroids for chronic rhinosinusitis | ||||||
|
Patient or population: chronic rhinosinusitis Intervention: systemic antibiotics (macrolide) Comparison: oral corticosteroids | ||||||
|
Outcomes № of participants (studies) |
Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without systemic antibiotics | With systemic antibiotics | Difference | ||||
| Disease‐specific HRQL | The study did not report this as an outcome | |||||
| Disease severity score | The study did not report this as an outcome | |||||
| Gastrointestinal disturbances Follow‐up: 3 months № of participants: 28 (1 RCT) |
RR 1.00 (0.16 to 6.14) |
Study population | ⊕⊝⊝⊝ VERY LOW1 | It is very unclear whether there is a difference in gastrointestinal disturbances between the groups. | ||
| 143 per 1000 |
143 per 1000 (23 to 877) |
0 fewer per 1000 (120 fewer to 734 more) |
||||
| General health‐related quality of life | The study did not report this as an outcome | |||||
| Suspected allergic reaction (rash or skin irritation) Follow‐up: 3 months № of participants: 28 (1 RCT) |
RR 2.00 (0.20 to 19.62) |
Study population | ⊕⊝⊝⊝ VERY LOW1 | It is unclear whether there is a difference in skin irritation between the groups. | ||
| 71 per 1000 |
143 per 1000 (14 to 1000) |
71 more per 1000 (57 fewer to 1330 more) |
||||
| Anaphylaxis or other very serious reactions (e.g. Stevens‐Johnson syndrome)
Follow‐up: 3 months № of participants: 28 (1 RCT) |
No events were reported in either arm. The effect size could not be estimated. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;HRQL: health‐related quality of life; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded to very low quality due to limitations of study design (lack of information about randomisation, allocation concealment and blinding, high risk of reporting bias), indirectness of the included population and intervention (study included a population who were more severely affected, those with recurrent polyps or recalcitrant disease, and the intervention was a 20‐day course of antibiotics) and imprecision (small study, n = 28) with a low number of events leading to large confidence intervals.
Where the range of scales and values for minimal important differences (MID) were unclear, we used the standardised mean difference (SMD) as a guide to estimate the effect sizes. As suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), we used standard rules of thumb in the interpretation of effect sizes (SMD, or Cohen's effect size of < 0.41 = small, 0.40 to 0.70 = moderate, > 0.70 = large) (Cohen 1988).
Established scales such as the Sino‐Nasal Outcome Test‐22 (SNOT‐22) may have other known MID values (MID = 9 points for SNOT‐22) and we used those to guide our interpretation whenever available (Hopkins 2009). In the absence of a MID, we used other rules of thumb to estimate this (MID = 0.5 SMD) and to guide our interpretation whenever available (Jaeschke 1989; Revicki 2008).
Antibiotics versus placebo
See also Table 1.
Three studies reported on this comparison (Otten 1994; Van Zele 2010; Wallwork 2006). Van Zele 2010 (33 participants) compared a tetracycline (doxycycline) with a placebo in adults with massive bilateral nasal polyps or bilateral nasal polyps that had recurred after surgery. Wallwork 2006 (64 participants) compared a macrolide antibiotic (roxithromycin) with a placebo in adults with a history consistent with a diagnosis of chronic rhinosinusitis but without nasal polyps. The remaining paper (79 participants) compared treatment with a cephalosporin‐type antibiotic (cefaclor) with placebo in children with chronic sinusitis but did not report any outcomes relevant to this review (Otten 1994).
Primary outcomes
1. Disease‐specific health‐related quality of life
One study (64 participants) compared roxithromycin against placebo treatment using the SNOT‐20 instrument to assess quality of life (range: 0 to 5; 0 = least quality of life, 5 = most quality of life) at the end of treatment (three months) and three months after treatment had completed (six months) (Wallwork 2006). At the end of treatment people with chronic rhinosinusitis without nasal polyps receiving roxithromycin had an improved quality of life compared with the placebo group (mean difference (MD) ‐0.54, 95% confidence interval (CI) ‐0.98 to ‐0.10; 64 participants; one study). This mean difference equates to a moderate effect size (SMD = 0.62). At three months after treatment it is very uncertain if there is a difference between the two groups (MD ‐0.35, 95% CI ‐0.81 to 0.11; 64 participants; one study). The observed mean difference corresponds to a small effect size (SMD = 0.37) (Analysis 1.1).
1.1. Analysis.
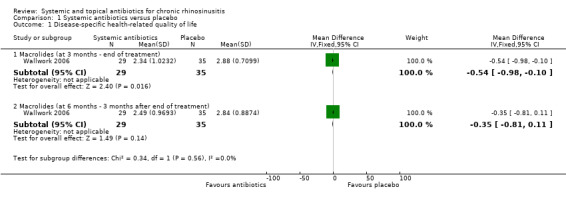
Comparison 1 Systemic antibiotics versus placebo, Outcome 1 Disease‐specific health‐related quality of life.
2. Disease severity, as measured by patient‐reported symptom score
One study in people with chronic rhinosinusitis with nasal polyps (33 participants) compared doxycyline (duration approximately 20 days) against placebo and measured the following patient‐assessed symptoms: anterior rhinorrhoea, nasal obstruction, post‐nasal drip and loss of sense of smell after treatment (20 days) and 12 weeks from the start of the trial (Van Zele 2010). Details of the scales used to record symptoms are not provided in the paper and the results are not sufficient to allow them to be used in a quantitative analysis. We contacted the author but they did not provide additional information.
3. Significant adverse effect: gastrointestinal disturbances
One study in people with chronic rhinosinusitis with nasal polyps (33 participants) compared a short course of doxycyline (duration approximately 20 days) against placebo and reported the adverse event of "reflux and/or gastric pain" at 12 weeks (Van Zele 2010). There were a low number of events in both arms: 2/14 in the doxycycline arm and 2/19 in the placebo arm (RR 1.36, 95% CI 0.22 to 8.50; 33 participants; one study) (Analysis 1.2).
1.2. Analysis.
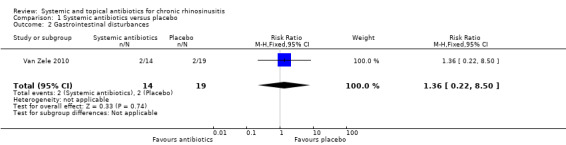
Comparison 1 Systemic antibiotics versus placebo, Outcome 2 Gastrointestinal disturbances.
Secondary outcomes
1. General health‐related quality of life
This was not reported as an outcome in any study.
2. Other adverse event: suspected allergic reaction (rash or skin irritation)
One study in people with chronic rhinosinusitis with nasal polyps (33 participants) reported the adverse event of "skin rash" at 12 weeks after a short course of doxycycline (duration approximately 20 days) (Van Zele 2010). There were very few events in both arms: 2/14 in the doxycycline arm and 0/19 in the placebo arm (RR 6.67, 95% CI 0.34 to 128.86; 33 participants; one study) (Analysis 1.3).
1.3. Analysis.
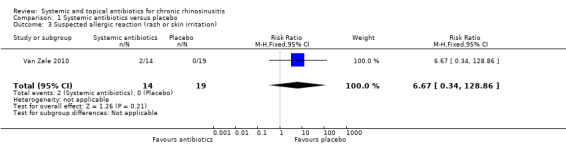
Comparison 1 Systemic antibiotics versus placebo, Outcome 3 Suspected allergic reaction (rash or skin irritation).
3. Other adverse event: anaphylaxis or other serious allergic reactions such as Stevens‐Johnson syndrome
One study in people with chronic rhinosinusitis with nasal polyps (33 participants) reported adverse events well and so we assumed that if an anaphylaxis event or other serious allergic reaction had occurred, this would have been reported in the paper (Van Zele 2010). We assumed that no events occurred in either arm of the study. No risk estimate could be provided.
4. Endoscopic scores (including nasal polyps score)
Two studies (97 participants) report the results of endoscopy (Van Zele 2010; Wallwork 2006). Van Zele 2010 (33 participants) compared doxycycline (duration approximately 20 days) with placebo in people with chronic rhinosinusitis with nasal polyps. They measured nasal polyps in each nostril on a five‐point scale (0 to 4; 0 = no polyps, 4 = large polyps). The scores for each nostril were then added together to get an overall nasal polyp score (final range 0 to 8, 0 = least severe). The paper presented the results graphically as change from baseline polyps score, however, despite contacting the author, data on the variance of the point estimates were not available and it was not possible to impute them. The paper states, "Administration of doxycycline for 20 days significantly reduced polyp size, starting at week 2, compared with placebo (visit 3; P = 0.005). The significant reduction of polyp size remained for up to 12 weeks after dosing (visit 4, P5.001; visit 5, P = 0.002; and visit 6, P = 0.015)."
Wallwork 2006 (64 participants) presented the results of an overall endoscopy score in patients with chronic rhinosinusitis without nasal polyps. They graded swelling (0 to 2), mucosal colour (0 to 1), polyps in the middle meatus (0 to 1) and nasal secretions (0 to 3). The final range of values was between 0 and 7 (0 = least severe, 7 = most severe). The results at three months were: MD ‐0.30 (95% CI ‐0.85 to 0.25; 64 participants; one study). The observed mean difference corresponds to a small effect size (SMD = 0.27) (Analysis 1.4).
1.4. Analysis.
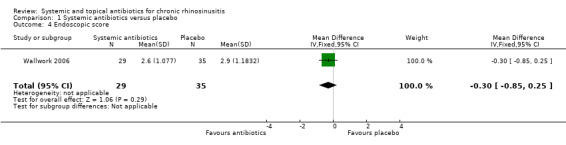
Comparison 1 Systemic antibiotics versus placebo, Outcome 4 Endoscopic score.
We did not present endoscopy scores in the 'Summary of findings' table as we did not consider this to be a priority outcome.
5. Computerised tomography (CT) scan score
This was not reported as an outcome in any of the studies.
Antibiotics plus saline plus intranasal corticosteroids versus placebo plus saline plus intranasal corticosteroids
See also Table 2.
One study (60 participants) reported on this comparison (Videler 2011). The population included people with chronic rhinosinusitis both with and without nasal polyps. The participants were a more severely affected population who had not responded to standard treatment. The study compared a macrolide antibiotic (azithromycin) for 12 weeks against placebo. All participants in both arms used nasal saline irrigation twice daily and the data suggest that 70% of patients also received intranasal steroids.
Primary outcomes
1. Disease‐specific health‐related quality of life
Videler 2011 reported results for health‐related quality of life at 14 weeks (53 participants) and six months (51 participants) using the SNOT‐22 outcome measure (range: 0 to 110, 0 = least affected, 110 = most affected). There was a potentially clinically important difference in the baseline SNOT‐22 score between the two groups (antibiotics score = 48.2, placebo score: 40.0, known MID 8.9 points (Hopkins 2009)). The studies reported both the scores and change from baseline at 14 weeks (two weeks after the end of antibiotic treatment) and at six months (three months after the end of antibiotic treatment).
At 14 weeks (two weeks after the end of antibiotic treatment) the mean change from baseline score was ‐3.7 (standard deviation (SD) 16.7) in the antibiotics group and ‐8.9 (SD 15.6) in the placebo group. These data are highly skewed and the authors reported no statistically significant difference using the Mann Whitney U‐test (P value = 0.3).
At six months (three months after the end of antibiotic treatment) the mean change from baseline score was ‐8.5 (SD 20.3) in the antibiotics group and ‐5.2 (SD 18.9) in the placebo group (Mann Whitney U‐test P value = 0.528).
Since the data were highly skewed and parametric tests did not detect a statistically significant difference, it is uncertain whether there is a difference between groups.
2. Disease severity, as measured by patient‐reported symptom score
Global change of symptom score
The study used a "Patient Response Rating Scale" (validation not reported) to classify the subjective effect of the course (‐2 desperately worse (deterioration of symptoms with significant impact on normal life); ‐1 worse (compared with the pretreatment situation); 0 no change; 1 improvement (although symptoms are present, they are scarcely troublesome); and 2 cured (virtually no symptoms present)). We dichotomised the score into the proportion of patients who had improved (categorising patients who scored "improvement" and "cured" as improved). There was no difference between the antibiotics and placebo groups; the risk ratio for improvement was 1.50 (95% CI 0.81 to 2.79; 56 participants; one study) (Analysis 2.1). The quality of the evidence is very low.
2.1. Analysis.
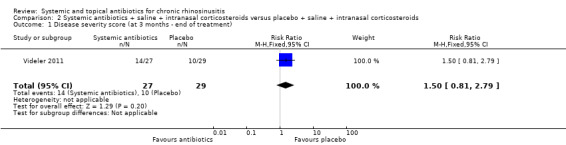
Comparison 2 Systemic antibiotics + saline + intranasal corticosteroids versus placebo + saline + intranasal corticosteroids, Outcome 1 Disease severity score (at 3 months ‐ end of treatment).
Individual symptoms
Videler 2011 (56 participants) asked patients to report symptoms on a 0 to 10 VAS (0 = no complaints, 10 = worst possible symptoms) for the following individual symptoms: headache, nasal obstruction, rhinorrhoea, post‐nasal drip, feeling of fullness, smell disturbance, facial pain, toothache, tears, coughing, nasal bleeding and crusts. The paper presents the mean change from baseline in the symptoms scores after treatment (12 weeks). However, the results were very highly skewed and only the mean and SD were reported. The study reported no statistically significant difference using the Mann Whitney U test. We are uncertain whether there was a difference at the end of the three‐month treatment course for any of the individual symptoms.
Nasal obstruction: mean change in azithromycin group ‐1.1 (SD 3.6); mean change in placebo group ‐1.4 (SD 2.9).
Rhinorrhoea: mean change in azithromycin group ‐0.7 (SD 3.1); mean change in placebo group ‐0.7 (SD 2.2).
Facial pain: mean change in azithromycin group 0.7 (SD 3.3); mean change in placebo group ‐0.6 (SD 2.5).
Loss of sense of smell: mean change in azithromycin group ‐0.4 (SD 3.5); mean change in placebo group ‐0.9 (SD 3.2).
None of the results for individual symptoms are presented in the GRADE 'Summary of findings' table as we did not consider this to be a priority outcome.
3. Significant adverse effect: gastrointestinal disturbances
Videler 2011 (56 participants) presented information on the number of people who reported "mild gastrointestinal complaints, mostly mild diarrhoea". The denominator is not clear for this outcome and so we used the denominators at 12 weeks in the analysis. There was no demonstrable difference in the rate of gastrointestinal disturbances between the groups (RR 1.07, 95% CI 0.16 to 7.10; 56 participants; one study) (Analysis 2.2).
2.2. Analysis.
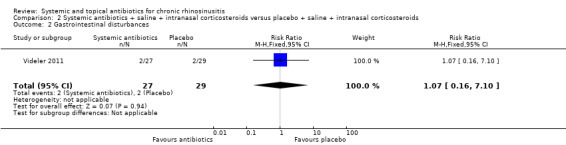
Comparison 2 Systemic antibiotics + saline + intranasal corticosteroids versus placebo + saline + intranasal corticosteroids, Outcome 2 Gastrointestinal disturbances.
Secondary outcomes
1. General health‐related quality of life
One study asked participants (56) to complete the SF‐36 generic quality of life measure before and after treatment (Videler 2011). The paper presented a table of the calculated P values, comparing the antibiotic group with the placebo group for the different domains of the SF‐36. The study authors noted that there were no statistically significant results for any of the domains at 12 weeks (end of treatment) or at 14 weeks (two weeks after the end of treatment).
2. Other adverse event: skin suspected allergic reaction (rash or skin irritation)
Videler 2011 stated that "No serious adverse events were reported during the use of trial medication other than mild gastrointestinal complaints...". It is not clear whether skin irritation would have been reported.
3. Other adverse event: anaphylaxis or other serious allergic reactions such as Stevens‐Johnson syndrome
Videler 2011 stated that "No serious adverse events were reported during the use of trial medication other than mild gastrointestinal complaints...", so we assumed that if an anaphylaxis event or other serious allergic reactions had occurred, this would have been reported in the paper. As no events were reported in either arm no risk estimate can be provided.
4. Endoscopic scores (including nasal polyps score)
Videler 2011 presented endoscopic score results. All patients underwent endoscopy and the following elements were graded:
mucosal colour (0 to 1) (left and right);
mucosal swelling (0 to 2) (inferior and middle turbinate; both sides);
nasal secretions (0 to 1);
crusts (0 to 2);
polyps (0 to 2) (inferior, middle meatus and ethmoid area; both sides); and
postnasal drip (0 to 1).
The total range of final values was 0 to 25 (0 = least severe, 25 = most severe). The full results were not reported in the paper. The paper stated that "one item showed a significant difference in the chi‐square test for trend. Secretion is the left middle meatus improved more in the antibiotic group". No significant differences were found for any of the other items.
5. Computerised tomography (CT) scan score
This was not reported as an outcome.
Antibiotics versus intranasal corticosteroids
One study compared a systemic macrolide antibiotic (clarithromycin) with intranasal steroids (mometasone furoate) in 43 Chinese people with chronic rhinosinusitis without nasal polyps (Zeng 2011).
Primary outcomes
1. Disease‐specific health‐related quality of life
This was not reported as an outcome in the study.
2. Disease severity, as measured by patient‐reported symptom score
One study in people with chronic rhinosinusitis without nasal polyps reported this outcome (Zeng 2011). The trial participants (43) reported individual symptoms on a 0 to 10 visual analogue scale (VAS) (0 = no complaints whatsoever, 10 = worst imaginable complaints) at baseline and at the end of the treatment (three months). The following symptoms were included: nasal obstruction, headache, facial pain, loss of sense of smell and rhinorrhoea. We added together the results for the four symptoms representing the EPOS 2012 criteria (nasal obstruction, rhinorrhoea, loss of sense of smell and facial pain) to create the mean total symptom score (range 0 to 40, 0 = least complaints) in the antibiotics and intranasal corticosteroids group (see Dealing with missing data for the methods used on the results). It is very uncertain whether there is a difference between the groups as patient‐reported disease severity was similar (MD ‐0.32, 95% CI ‐2.11 to 1.47; 43 participants; one study) (Analysis 3.1). The observed mean difference corresponds to a small effect size (SMD = 0.11).
3.1. Analysis.
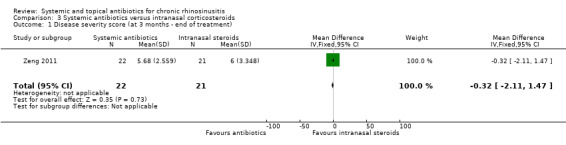
Comparison 3 Systemic antibiotics versus intranasal corticosteroids, Outcome 1 Disease severity score (at 3 months ‐ end of treatment).
Individual symptom scores
Zeng 2011 also provided the results for the individual symptom scores measured as described above at the end of the three‐month treatment course. The results are presented in Analysis 3.2 and below.
3.2. Analysis.
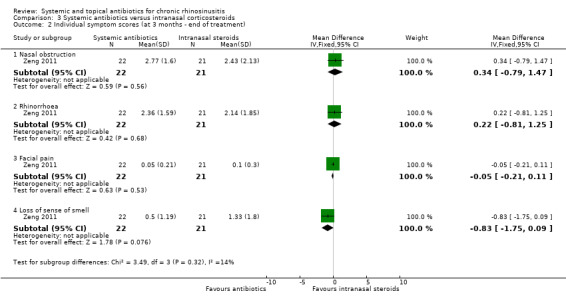
Comparison 3 Systemic antibiotics versus intranasal corticosteroids, Outcome 2 Individual symptom scores (at 3 months ‐ end of treatment).
Nasal obstruction: MD 0.34, 95% CI ‐0.79 to 1.47; 43 participants; one study.
Rhinorrhoea: MD 0.22, 95% CI ‐0.81 to 1.25; 43 participants; one study.
Facial pain: MD ‐0.05, 95% CI ‐0.21 to 0.11; 43 participants; one study.
Loss of sense of smell: MD ‐0.83, 95% CI ‐1.75 to 0.09; 43 participants; one study.
None of the results for individual symptoms are presented in the GRADE 'Summary of findings' table as we considered it to be re‐presenting information that was already included in the disease severity score.
3. Significant adverse effect: gastrointestinal disturbances
Zeng 2011 did not mention adverse events.
Secondary outcomes
1. General health‐related quality of life
This was not reported as an outcome.
2. Other adverse event: skin suspected allergic reaction (rash or skin irritation)
Zeng 2011 did not mention adverse events.
3. Other adverse event: anaphylaxis or other serious allergic reactions such as Stevens‐Johnson syndrome
Zeng 2011 did not mention adverse events.
4. Endoscopic scores (including nasal polyps score)
One study (43 participants) reported this outcome for people with chronic rhinosinusitis without nasal polyps (Zeng 2011). Endoscopic appearance was measured using the Lanza‐Kennedy score, which assesses discharge (0 to 2, 0 = no discharge) and swelling (0 to 2, 0 = no swelling) on each side separately at the end of treatment (three months). The scores from each side were then added. The paper presents the results for discharge and swelling separately.
Discharge: The discharge result at 12 weeks after dosing in the antibiotics group was 0.32 ± 0.57 and for the intranasal steroids group was 0.71 ± 1.23.
Swelling: The swelling result at 12 weeks after dosing in the antibiotics group was 0.23 ± 0.61 and for the intranasal steroids group was 0.62 ± 0.92.
Total score: The discharge and swelling scores were combined to given the overall endoscopic score (range: 0 to 8; 0 = least severe). One study (43 participants) gave results at 12 weeks after dosing (MD ‐0.78, 95% CI ‐1.52 to ‐0.04; 43 participants; one study) (Analysis 3.3). The observed mean difference corresponds to a moderate effect size (SMD = 0.62).
3.3. Analysis.
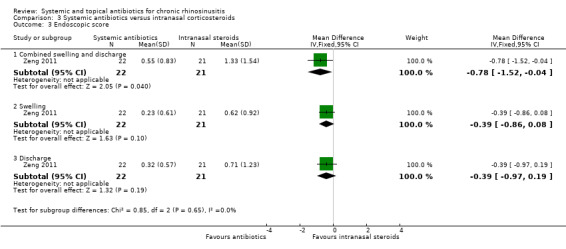
Comparison 3 Systemic antibiotics versus intranasal corticosteroids, Outcome 3 Endoscopic score.
We did not present endoscopy scores in the 'Summary of findings' table as we did not consider it to be a priority outcome.
5. Computerised tomography (CT) scan score
This was not reported as an outcome.
Adverse events related to intranasal corticosteroids use
Adverse events relating to intranasal steroid use were identified in Chong 2016a and Chong 2016b as epistaxis, local irritation and osteoporosis. Zeng 2011 did not mention any adverse events related to the use of intranasal steroids.
Antibiotics versus oral steroids
One study (28 participants) compared a tetracycline (doxycycline) (duration approximately 20 days) with a 20‐day course of oral steroids (methylprednisolone) in adults with massive bilateral nasal polyps or bilateral nasal polyps that had recurred after surgery (Van Zele 2010).
Primary outcomes
1. Disease‐specific health‐related quality of life
This was not reported as an outcome.
2. Disease severity, as measured by patient‐reported symptom score
Van Zele 2010 (28 participants), in people with chronic rhinosinusitis with nasal polyps, measured the following patient‐assessed symptoms: anterior rhinorrhoea, nasal obstruction, post‐nasal drip and loss of sense of smell after treatment (20 days) and 12 weeks from the start of the trial. Details of the scales used to record symptoms are not provided in the paper and the results in the paper are not sufficient to allow them to be used. We contacted the author but they did not provide additional information.
3. Significant adverse effect: gastrointestinal disturbances
One study (28 participants), in people with chronic rhinosinusitis with nasal polyps, reported the adverse event of "reflux and/or gastric pain" at 12 weeks (Van Zele 2010). There were a low number of events in both arms: 2/14 in the doxycycline arm and 2/14 in the oral steroids arm (RR 1.00, 95% CI 0.16 to 6.14; 28 participants; one study) (Analysis 4.1).
4.1. Analysis.
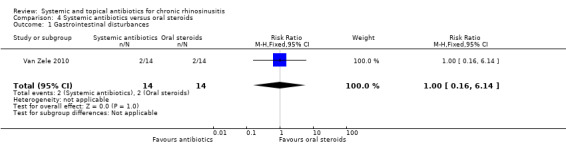
Comparison 4 Systemic antibiotics versus oral steroids, Outcome 1 Gastrointestinal disturbances.
Secondary outcomes
1. General health‐related quality of life
This was not reported as an outcome.
2. Other adverse event: skin suspected allergic reaction (rash or skin irritation)
One study (28 participants) in people with chronic rhinosinusitis with nasal polyps reported the adverse event of "skin rash" at 12 weeks (Van Zele 2010). There were very few events in both arms: 2/14 in the doxycycline arm and 1/14 in the placebo arm (RR 2.00, 95% CI 0.20 to 19.62; 28 participants; one study) (Analysis 4.2).
4.2. Analysis.
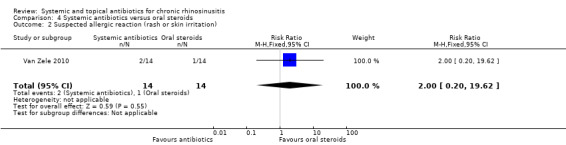
Comparison 4 Systemic antibiotics versus oral steroids, Outcome 2 Suspected allergic reaction (rash or skin irritation).
3. Other adverse event: anaphylaxis or other serious allergic reactions such as Stevens‐Johnson syndrome
One study (28 participants) in people with chronic rhinosinusitis with nasal polyps reported adverse events well and so we assumed that if an anaphylaxis event or other serious allergic reaction had occurred, this would have been reported in the paper (Van Zele 2010). No events were reported in either arm so no risk estimate could be calculated.
4. Endoscopic scores (including nasal polyps score)
One study in people with chronic rhinosinusitis with nasal polyps measured nasal polyps score on a five‐point scale (0 to 4), which was used for each nostril and then summed to get an overall nasal polyp score (Van Zele 2010). The paper presented the results graphically as change from baseline polyps score, however data on the variance of the point estimates were not available for this study and it was not possible to impute them from other studies due to differences in the scale. It was therefore not included in the analysis.
This outcome is not presented in the 'Summary of findings' table as we did not consider it to be a priority outcome.
5. Computerised tomography (CT) scan score
This was not reported as an outcome.
Adverse events related to oral steroid use
Adverse events related to oral steroid use were identified in the Cochrane reviews Head 2016a and Head 2016b as mood disturbances, insomnia and gastrointestinal disturbances. Of these, gastrointestinal disturbances have already been reported (Analysis 4.1). Mood disturbances were not reported as an adverse event in the study included in this section (Van Zele 2010). Insomnia was reported in one study (28 participants) but there was only one event reported in each arm (RR 1.00, 95% CI 0.07 to 14.45; 28 participants; one study).
Discussion
Summary of main results
Five studies are included in this review, with a total of 293 randomised participants. These studies provide evidence for four different comparisons. There was variation in the trials regarding the type of antibiotics used, the duration of treatment, the concomitant treatments and the outcomes measured.
Antibiotics versus placebo
Three studies compared antibiotic treatment with placebo (176 participants).
Disease‐specific health‐related quality of life (HRQL) was reported using the SNOT‐20 (0 to 5, 5 = worst quality of life) by one study in adults without nasal polyps (64 participants). At the end of treatment, the SNOT‐20 score was lower in the group receiving three months of treatment with macrolide antibiotics than the placebo group (mean difference (MD) ‐0.54 points, 95% confidence interval (CI) ‐0.98 to ‐0.10). However, three months after treatment it was uncertain whether there was a difference in effect between the groups. We assessed the evidence to be of moderate quality (GRADE).
One study in people with polyps (33 participants) provided information on gastrointestinal disturbances and suspected allergic reaction (rash or skin irritation) 10 weeks after a short course of tetracycline antibiotic treatment (duration ˜20 days) compared with placebo treatment. It was very uncertain whether there was an increase in gastrointestinal disturbances in the antibiotics group (risk ratio (RR) 1.36, 95% CI 0.22 to 8.50; very low quality evidence) or skin irritation (RR 6.67, 95% CI 0.34 to 128.86; very low quality evidence).
None of the studies presented information on patient‐reported disease severity or general health‐related quality of life.
Antibiotics and saline irrigation versus placebo and saline irrigation
One study (60 participants) compared a three‐month treatment course of macrolide antibiotic with placebo; all participants also used nasal saline irrigation and 70% used intranasal corticosteroids. Disease‐specific HRQL was reported using the SNOT‐22 (0 to 110, 110 = worst quality of life). The data for this outcome were highly skewed (medians not reported) and there was baseline imbalance between the groups that could be of clinical importance. The data were difficult to interpret and it is unclear whether there was an important difference between groups after treatment. We assessed the quality of the evidence to be low. To assess patient‐reported disease severity the study authors asked participants to measure the effect of the treatment course on a five‐point scale (‐2 desperately worse to 2 cured) at the end of treatment (three months). We dichotomised this to give a proportion of patients who had improved (considering patients who scored "improvement" and "cured" as improved). There was no difference between the antibiotics and placebo groups; the risk ratio for improvement in symptoms was 1.50 (95% CI 0.81 to 2.79; 56 participants; one study) and there was also a slightly higher number of people who felt worse after the treatment. We assessed the quality of the evidence to be very low.
There was no demonstrable difference in the rate of gastrointestinal disturbances between the groups (RR 1.07, 95% CI 0.16 to 7.10). General HRQL was measured using the SF‐36 instrument. The full results were not reported but the authors state that there was no difference between the two groups at the end of treatment (12 weeks) or two weeks later.
Antibiotics versus intranasal steroids
One study (43 participants) compared a three‐month treatment course of macrolide antibiotic with intranasal corticosteroids in people without polyps. Patient‐reported disease severity was assessed using a composite symptom score (range: 0 to 40; 0 = no symptoms). It was very uncertain whether the effect of antibiotics differed from intranasal steroids as patient‐reported disease severity was similar (MD ‐0.32, 95% CI ‐2.11 to 1.47; low quality evidence).
Antibiotics versus oral steroids (one study)
One study (28 participants) in people with nasal polyps compared a short course of tetracycline antibiotic treatment (unclear duration, ˜20 days) with a 20‐day course of oral corticosteroids. We were unable to extract data on any of the primary or secondary efficacy outcomes from the paper. It was very uncertain whether there was an increase in gastrointestinal disturbances (RR 1.00, 95% CI 0.16 to 6.14) or skin irritation (RR 2.00, 95% CI 0.20 to 19.62) as the results for these outcomes were similar in both groups. We assessed this evidence to be of very low quality.
Overall completeness and applicability of evidence
There were only five studies that met the inclusion criteria for this review. All of these studies were small (43 to 79 participants) and they covered four different comparisons. Other differences between the studies include the following:
Age of participants: four studies recruited adults (Van Zele 2010; Videler 2011; Wallwork 2006; Zeng 2011); one only included children (Otten 1994).
Nasal polyps: three studies only included people without nasal polyps (Otten 1994; Wallwork 2006; Zeng 2011), one a mix of people with and without nasal polyps where the results were not presented separately (Videler 2011), and the last study only included patients with nasal polyps (Van Zele 2010)
Antibiotics: each of the studies used different antibiotics. Of these, three belonged to the macrolide class (roxithromycin (Wallwork 2006), azithromycin (Videler 2011), clarithromycin (Zeng 2011)), one was a tetracycline (doxycycline (Van Zele 2010)), and the last was a cephalosporin‐type antibiotic (cefaclor (Otten 1994)).
Duration of antibiotics: whilst three studies investigated longer courses of antibiotics for up to three months of treatment, one study looked only at a short course (approximately 20 days), but followed patients up for 10 weeks after the initial treatment had ended (Van Zele 2010).
Comparisons: three studies compared antibiotics against placebo (Otten 1994; Van Zele 2010; Wallwork 2006), one against intranasal steroids (Zeng 2011), and one against oral steroids (Van Zele 2010). The remaining study used antibiotics as an adjuvant, on top of nasal saline irrigation, and 70% of participants also used intranasal corticosteroids (Videler 2011).
When the differences between the studies are considered, it is difficult to draw overall conclusions for this review. This underlines the issues we encountered with the paucity of reporting of trial data, as presented here.
The restriction of study inclusion only to those that had a minimum duration of follow‐up of three months may have had a big impact on the evidence that we found to answer this review question. We chose this cut‐off point so that the results of the review would reflect the impact of treatment on medium‐term outcomes in chronic rhinosinusitis patients. There were potentially 11 additional studies where the follow‐up period was between 4 and 12 weeks, although some of these studies may have been reporting acute sinusitis. Although the inclusion of these studies may have added to the evidence base, it should be remembered that chronic rhinosinusitis is a chronic condition, the definition of which means that patients will have experienced symptoms for more than 12 weeks. Reviewing the evidence for the effects of interventions in people who have been followed up for less than 12 weeks could potentially be misleading with regard to the long‐term consequences of treatment and whether any benefits are sustained: it would reflect a shorter‐term view of the impact of treatment. However, this is an issue that could be considered in future updates of the review.
Adverse events were generally poorly reported in the studies. Of the five studies included, three did not mention adverse events (Otten 1994; Wallwork 2006; Zeng 2011). The remaining two presented results for adverse events but did not provide details of how the events were defined or the methods of collection (Van Zele 2010; Videler 2011). None of the studies were powered to identify adverse events such as allergic reactions and so it is not surprising that no such events were reported. Caution should be applied when interpreting the adverse events results as this does not mean that there is no difference between the two groups. In addition, none of the studies were set up to investigate other important factors related to the extended use of antibiotics, such as shifts in bacterial flora and increase in subsequently induced bacterial resistance.
Elevated serum immunoglobulin (IgE) levels are seen in patients with atopic disease. It has been suggested that IgE might be a surrogate for an eosinophilic subgroup of chronic rhinosinusitis and thus a non‐responder to the anti‐neutrophilic effects of macrolides (Harvey 2009). Patients with eosinophilic disease have previously been noted to be poor responders (Haruna 2009). One included study showed a significant improvement in the endoscopic score and the SNOT‐20 score at the end of treatment (12 weeks) in those patients with a low serum‐IgE (Wallwork 2006). However, this has not been corroborated by other studies. Zeng 2011 did not find an influence of atopy on the therapeutic effect of clarithromycin and so this factor clearly requires further investigation.
Quality of the evidence
With the exception of the evidence for health‐related quality of life in the comparison of antibiotics with placebo (which we assessed to be of moderate quality), the quality of the evidence for all of the other outcomes assessed was low or very low, including for the adverse events outcomes that were reported.
The quality of the evidence was affected by a number of issues: methodological limitations, the lack of validation of outcome instruments, the directness of the population included and the sample sizes of the studies. Some of the studies were poorly reported and lacked information about randomisation, allocation concealment and blinding. Where the results for disease‐specific quality of life were presented the studies had generally used validated instruments. However, for the other patient‐reported outcomes, specifically symptom severity scores, the studies did not provide information on instrument validation. The variety of chronic rhinosinusitis populations included in the studies was wide and it is unclear how the results of studies in participants with nasal polyps that have recurred after surgery (Van Zele 2010), or the results from a population without nasal polyps who have never had surgery (Zeng 2011), can be applied to the general chronic rhinosinusitis population.
Lastly, the size of the studies, and consequently the precision of the results, was one of the biggest factors affecting study quality. The average size of the studies included in this review was 59 participants in total. This limits how much confidence can be placed in the results.
Potential biases in the review process
At the protocol stage we identified the validation of outcome measures as a potential bias that could affect the validity of the results (Chong 2015). Many of the studies did not use patient‐reported symptom scales that had been appropriately validated. The lack of validated scores meant that we were required to make judgements based on the face validity of the scale, rather than having reliable validity data. Even where validated scales had been used these may have missed elements that are key to the quality of life of people with chronic rhinosinusitis. For example, the SNOT‐20 questionnaire omits nasal blockage and smell function (which are included in the SNOT‐22 instrument). As validated disease‐specific questionnaires exist, future trials would benefit from using these as primary outcome measures. Recent preliminary work in the UK has underlined this and identified the need to establish a core outcome set for rhinosinusitis (Hopkins 2016).
Due to the lack of outcomes reported using validated measures, in order to enable some comparison between studies we took the decision to combine the scores for individual symptoms to create a total symptom score. The methods we used to do this are described in the methods section (Dealing with missing data). The symptoms included were based on the EPOS 2012 diagnostic criteria, but the score calculated was not a validated measure and the correlation between symptoms was not accounted for in the results. This may have had an effect on the magnitude of the effect size.
Agreements and disagreements with other studies or reviews
The current review updates a previous Cochrane review (Piromchai 2011). It increases the scope of the review to include both adults and children, patients both with and without nasal polyps, and both systemic and topical antibiotics. The previous Cochrane review included one study (Wallwork 2006), which is also included in this review. Two studies included in this review were published after the publication date of the previous review (Videler 2011; Zeng 2011). Two additional studies have been included due to the population inclusion criteria being widened to include children (Otten 1994), and people with nasal polyps (Van Zele 2010). Despite the inclusion of additional trials the conclusion regarding the lack of evidence for this intervention is still valid.
The EPOS 2012 guidelines separated the evidence into those with and those without nasal polyps, and then each category into three sections: short‐term systemic antibiotics (less than four weeks), long‐term systemic antibiotics and topical antibiotics.
For patients with nasal polyps, EPOS 2012 identified two studies investigating short‐term antibiotics (Schalek 2009; Van Zele 2010). We included Van Zele 2010 in this review but we excluded Schalek 2009 as all of the patients in the study underwent functional endoscopic sinus surgery (FESS) during the trial. They concluded that there may have been a small improvement in polyp size and postnasal discharge but that the evidence for improved quality of life was lacking. For people with chronic rhinosinusitis with nasal polyps, the EPOS 2012 document included three papers for long‐term antibiotics, all of which were open studies that we excluded from this review due to the study design. They concluded that there may have been an improvement in patient symptoms and polyp size with antibiotics but that the clinical benefits had not been fully investigated. There were no studies identified that investigated topical antibiotics in patients with nasal polyps, which met the inclusion criteria.
For patients without nasal polyps, EPOS 2012 identified three studies that investigated short‐term use of antibiotics (Huck 1993; Legent 1994; Namyslowski 1998), all of which compared different antibiotics (not placebo‐controlled) and that we excluded from this review as the follow‐up duration was less than three months. Their conclusion was that short‐term use was probably only relevant for exacerbations with a positive culture. For long‐term antibiotics, the EPOS 2012 document reviewed the evidence for the use of macrolides in lower airway populations (diffuse panbronchiolitis and cystic fibrosis). They also identified two randomised controlled trials (Videler 2011; Wallwork 2006), both of which we included in this review, and seven open studies in the chronic rhinosinusitis population, which we excluded due to study design. The report concluded that "long‐term antibiotic treatment should be reserved for patients where nasal corticosteroids and saline irrigation has failed to reduce symptoms to an acceptable level". For topical antibiotics, the EPOS 2012 document identified three placebo‐controlled trials. We excluded all three from this review, two due to the follow‐up period (Desrosiers 2001; Sykes 1986), and one because the timing of the outcome assessment was unclear, although it was likely to be less than 12 weeks (Videler 2008). They concluded that topical antibacterial therapy could not be recommended in the treatment of chronic rhinosinusitis.
We identified one further well‐conducted, recent systematic review of macrolide therapy for chronic rhinosinusitis (Pynnonen 2013). This review found three studies, two of which we also included in our review (Videler 2011; Wallwork 2006), and one study that we excluded due to the duration of follow‐up (three‐week treatment with eight‐week follow‐up) (Amini 2009). This review analysed all three studies together, despite differences in the background treatment (both participant groups receiving saline and intranasal corticosteroids in Videler 2011), but concluded that no clinically significant difference in patient‐orientated outcomes could be identified. However, they did raise the potential for there being a subgroup of patients that may respond better (those with low serum IgE) and suggested this as an aspect that merited more research.
Authors' conclusions
Implications for practice.
We found very little evidence that systemic antibiotics are effective in patients with chronic rhinosinusitis. We did find moderate quality evidence of a modest improvement in disease‐specific quality of life in patients with chronic rhinosinusitis without nasal polyps receiving three months of a macrolide antibiotic. However, this improvement was small (0.5 points on a five‐point scale) and only seen at the end of the three‐month course of treatment; by three months later no effect was found.
It is unclear whether or not patients benefit from the use of antibiotics when they are also using saline irrigation and intranasal corticosteroids; the quality of this evidence is very low.
Despite a general understanding that the use of antibiotics can be associated with adverse effects, including gastrointestinal disturbance, the results for adverse events in this review were very uncertain as the number of participants in all the studies was small and few events were reported.
We found no studies of topical antibiotics that met the inclusion criteria.
Implications for research.
The evidence (up to September 2015) for antibiotics in people in chronic rhinosinusitis is of low quality, as we are uncertain about the estimates. The evidence suggests that antibiotic treatment for patients who have chronic rhinosinusitis may be beneficial in reducing the size of the polyps, but that although symptoms may be reduced in the short term compared to placebo this may not be sustained. The evidence is from very small studies (fewer than 80 patients each), with a variety of populations, interventions and comparisons. There are no well‐reported data on adverse effects or longer‐term outcomes, which are important to provide information on whether there is sustained benefit.
Future studies should be designed as adequately powered, double‐blind, randomised controlled trials and include patients with chronic rhinosinusitis diagnosed using the EPOS 2012 criteria. Trials should include both patients with and without nasal polyps (with stratification by subgroup). This should include patients whose symptoms have been refractory to treatment with saline irrigation and intranasal steroids for at least three months.
With regard to the intervention, the macrolide class of antibiotics is likely to yield the greatest benefit because these antibiotics have both anti‐inflammatory and antimicrobial properties, as demonstrated in other respiratory tract disorders (Donath 2013; Shi 2014), and they should provide good coverage of typical chronic rhinosinusitis flora (Genoway 2011). A number of publications have, however, raised concerns about cardiac toxicity with erythromycin in patients with a prolonged QT interval, and the most recent evidence has also implicated clarithromycin (Iyer 2016). However, in a chronic rhinosinusitis population, clarithromycin has a reasonable side effect profile, as seen in a recent feasibility study (Bewick 2014). There is still a case for placebo as the first choice of comparator as the evidence for the efficacy of antibiotics compared to placebo is not conclusive; however, surgical intervention may not be an unreasonable alternative comparator. Attention should be paid to any concurrent treatments given and these should be decided at the protocol stage.
The primary outcomes should be relevant to patients and any disease‐specific instruments should be validated in people with chronic rhinosinusitis. The methods for defining and recording adverse events should be considered at the protocol stage and the adverse events recorded should include gastrointestinal disturbances, skin rashes, dysgeusia, hepatoxicity and insomnia. The age of patients should be taken into account. Endoscopic evaluation should not be chosen as a primary outcome because the correlation between endoscopic results and patient symptoms is unclear. All outcomes should be reported at longer‐term follow‐up (a minimum of three months and ideally six months). Much longer‐term time points should also be considered (between six months and five years) (Soler 2010).
This review is one of a suite of reviews of medical treatments for chronic rhinosinusitis, each of which features its own research recommendations. Across all reviews, key features of future research are as follows:
Trials should be adequately powered and imbalances in prognostic factors (for example, prior sinus surgery) must be accounted for in the statistical analysis.
Study participants should be diagnosed with chronic rhinosinusitis using the EPOS 2012 criteria and should primarily be recruited based on their symptoms. Different patient phenotypes (that is, those with and without nasal polyps) should be recognised and trials should use stratified randomisation within these subgroups or focus on one or other of the phenotypes.
Studies should focus on outcomes that are important to patients and use validated instruments to measure these. Validated chronic rhinosinusitis‐specific health‐related quality of life questionnaires exist, for example the Sino‐Nasal Outcome Test‐22 (SNOT‐22). Patients may find dichotomised outcomes easiest to interpret; for example the percentage of patients achieving a minimal clinically important difference (MCID) or improvement for that outcome. Such MCIDs or cut‐off points should be included in the study protocol and clearly outlined in the methods section.
Trials and other high‐quality studies should use consistent outcomes and adhere to reporting guidelines, such as CONSORT, so that results can be compared across future trials. The development of a standardised set of outcomes, or core outcome set, for chronic rhinosinusitis, agreed by researchers, clinicians and patients, will facilitate this process.
Acknowledgements
This project is one of a suite of reviews on the medical treatment of chronic rhinosinusitis, funded by the National Institute for Health Research (award reference 14/174/03).
This project was also supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We would like to express our thanks to the external peer reviewer, Professor Wytske Fokkens, the consumer referee Joan Blakley and the Cochrane ENT editors for their detailed and insightful comments, which helped to strengthen this review. Thank you also to acting Co‐ordinating Editor, Professor Richard Harvey, for his oversight of this publication.
We are grateful to Linda Ware for her assistance with screening abstracts, and Dr. Takashi Fujiwara and Professor Motokazu Yanagi for completing the data extraction for Hashiba 1997.
We are indebted to Anna Kashchuk, Irina Telegina, Chungie Li, Anja Lieder and Helene Moustgaard for translating and excluding primary studies for this review.
The authors are grateful for the assistance provided by Jenny Bellorini and Samantha Faulkner, with editorial support and searching for studies.
Appendices
Appendix 1. Search strategies
| CENTRAL | Ovid MEDLINE |
| #1 MeSH descriptor: [Sinusitis] explode all trees #2 MeSH descriptor: [Rhinitis] this term only #3 MeSH descriptor: [Rhinitis, Atrophic] this term only #4 MeSH descriptor: [Rhinitis, Vasomotor] this term only #5 MeSH descriptor: [Paranasal Sinus Diseases] this term only #6 MeSH descriptor: [Paranasal Sinuses] explode all trees #7 rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis #8 kartagener* near syndrome* #9 inflamm* near sinus* #10 (maxilla* or frontal*) near sinus* #11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 #12 MeSH descriptor: [Chronic Disease] explode all trees #13 MeSH descriptor: [Recurrence] explode all trees #14 chronic or persis* or recurrent* #15 #12 or #13 or #14 #16 #11 and #15 #17 CRSsNP #18 (sinusitis or rhinitis) near (chronic or persis* or recurrent*) #19 #16 or #17 or #18 #20 MeSH descriptor: [Nasal Polyps] explode all trees #21 MeSH descriptor: [Nose] explode all trees #22 MeSH descriptor: [Nose Diseases] explode all trees #23 #21 or #22 #24 MeSH descriptor: [Polyps] explode all trees #25 #23 and #24 #26 (nose or nasal or rhino* or rhinitis or sinus* or sinonasal) near (papilloma* or polyp*) #27 rhinopolyp* or CRSwNP #28 #19 or #20 or #25 or #26 or #27 #29 MeSH descriptor: [Anti‐Bacterial Agents] explode all trees #30 MeSH descriptor: [Antibiotic Prophylaxis] explode all trees #31 MeSH descriptor: [Lactams] explode all trees #32 MeSH descriptor: [Quinolones] explode all trees #33 MeSH descriptor: [Macrolides] explode all trees #34 MeSH descriptor: [Tetracyclines] explode all trees #35 ANTIBIOT* or ANTI next BIOT* or ANTIMICROBIAL* or ANTI next MICROBIAL* or BACTERIOCID* or ANTIBACTERIAL* or ANTI next BACTERIAL* #36 PENICILLIN* or AMOXICILLIN or AMPICILLIN or CLAVULANIC or AMOXICLAV or AUGMENTIN or TICARCILLIN or TIMENTIN or FLUCLOXACILLIN or FLUAMPICIL or MAGNAPEN or PIPERACILLIN or TAZOCIN or CEPHALOSPORIN* or CEFACLOR or DISTACLOR or CEFADROXIL or BAXAN or CEFALEXIN or CEPOREX or KEFLEX or CEFAMANDOLE or KEFADOL or CEFAZOLIN* or KEFZOL or CEFIXIME or SUPRAX or CEFOTAXIME or CLAFORAN or CEFOXITIN or MEFOXIN or CEFPIROME or CEFROM or CEFPODOXIME or ORELOX or CEFPROZIL or CEFZIL or CEFRADINE or VELOSEL or CEFTAZIDIM or FORTUM or KEFADIM or CEFTRIAXONE or ROCEPHIN or CEFUROXIME or ZINACEF or ZINNAT or CEFONICID or AZTREONAM or AZACTAM or IMIPENEM or CILASTATIN or PRIMAXIN or MEROPENEM or TETRACYCLINE* or DETECLO or DEMECLEOCYCLIN or LEDERMYCIN or DOXYCYCLINE or VIBRAMYCIN or MINOCYCLINE or MINOCINE or OXYTETRACYCLINE or TERRAMYCIN or MACROLIDE* or ERYTHROMYCIN or ERYMAX or ERYTHROCIN or ERYTHROPED or AZITHROMYCIN or ZITHROMAX or CLARITHROMYCIN or KLARICID or TELITHROMYCIN or KETEK or TRIMOXAZOLE or SEPTRIN or TRIMETHOPRIM or MONOTRIM or TRIMOPAN or METRONIDAZOLE or FLAGYL or METROLYL or PHENOXYMETHYLPENICILLIN or SULFAMETHOXAZOLE or OXACILLIN or CEPHALOTHIN or SULBACTAM or OFLOXACIN or CLINDAMYCIN or GENTAMYCIN or VANCOMYCIN #37 cyclosporin* or Chlortetracycline or Lymecycline or Methacycline or Rolitetracycline or lactam* or quinolone* or Carbapenem* or Thienamycins or cephalosporin* or cefamandole or Cefazolin or Cefonicid or Cefsulodin or Cephacetrile or Cephalexin or Cephaloridine or Cephamycin* or Monobactam* or Aztreonam or Moxalactam or Amdinocillin or Cyclacillin or Methicillin or Nafcillin or Oxacillin or Sulbactam #38 Nalidixic or Nedocromil or Oxolinic or Carteolol or Fluoroquinolones or Ciprofloxacin or Enoxacin or Norfloxacin or Ofloxacin or Pefloxacin or Cofactor #39 Amphotericin or Antimycin or Brefeldin or Bryostatin* or Candicidin or Epothilone* or Ketolide* or Roxithromycin or Filipin or Ivermectin or Josamycin or Leucomycins or Kitasamycin or Spiramycin or Lucensomycin or Maytansine or Mepartricin or Miocamycin or Natamycin or Nystatin or Oleandomycin or Troleandomycin or Oligomycin* or Rutamycin or Sirolimus or Tacrolimus or Tylosin #40 #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 #41 #40 and #28 |
1 exp Sinusitis/ 2 paranasal sinus diseases/ or rhinitis/ or rhinitis, atrophic/ or rhinitis, vasomotor/ 3 exp Paranasal Sinuses/ 4 (rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis).ab,ti. 5 (kartagener* adj3 syndrome*).ab,ti. 6 (inflamm* adj5 sinus*).ab,ti. 7 ((maxilla* or frontal*) adj3 sinus*).ab,ti. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp chronic disease/ 10 exp Recurrence/ 11 (chronic or persis* or recurrent*).ab,ti. 12 9 or 10 or 11 13 8 and 12 14 CRSsNP.ab,ti. 15 ((sinusitis or rhinitis) adj3 (chronic or persis* or recurrent*)).ab,ti. 16 13 or 14 or 15 17 exp Nasal Polyps/ 18 exp Nose/ or exp Nose Diseases/ 19 exp Polyps/ 20 18 and 19 21 ((nose or nasal or rhino* or rhinitis or sinus* or sinonasal) adj3 (papilloma* or polyp*)).ab,ti. 22 (rhinopolyp* or CRSwNP).ab,ti. 23 16 or 17 or 20 or 21 or 22 24 exp Anti‐Bacterial Agents/ 25 exp Antibiotic Prophylaxis/ 26 exp Lactams/ 27 exp Quinolones/ 28 exp Macrolides/ 29 exp Tetracyclines/ 30 (ANTIBIOT* or ANTI next BIOT* or ANTIMICROBIAL* or ANTI next MICROBIAL* or BACTERIOCID* or ANTIBACTERIAL* or ANTI next BACTERIAL*).ab,ti. 31 (PENICILLIN* or AMOXICILLIN or AMPICILLIN or CLAVULANIC or AMOXICLAV or AUGMENTIN or TICARCILLIN or TIMENTIN or FLUCLOXACILLIN or FLUAMPICIL or MAGNAPEN or PIPERACILLIN or TAZOCIN or CEPHALOSPORIN* or CEFACLOR or DISTACLOR or CEFADROXIL or BAXAN or CEFALEXIN or CEPOREX or KEFLEX or CEFAMANDOLE or KEFADOL or CEFAZOLIN* or KEFZOL or CEFIXIME or SUPRAX or CEFOTAXIME or CLAFORAN or CEFOXITIN or MEFOXIN or CEFPIROME or CEFROM or CEFPODOXIME or ORELOX or CEFPROZIL or CEFZIL or CEFRADINE or VELOSEL or CEFTAZIDIM or FORTUM or KEFADIM or CEFTRIAXONE or ROCEPHIN or CEFUROXIME or ZINACEF or ZINNAT or CEFONICID or AZTREONAM or AZACTAM or IMIPENEM or CILASTATIN or PRIMAXIN or MEROPENEM or TETRACYCLINE* or DETECLO or DEMECLEOCYCLIN or LEDERMYCIN or DOXYCYCLINE or VIBRAMYCIN or MINOCYCLINE or MINOCINE or OXYTETRACYCLINE or TERRAMYCIN or MACROLIDE* or ERYTHROMYCIN or ERYMAX or ERYTHROCIN or ERYTHROPED or AZITHROMYCIN or ZITHROMAX or CLARITHROMYCIN or KLARICID or TELITHROMYCIN or KETEK or TRIMOXAZOLE or SEPTRIN or TRIMETHOPRIM or MONOTRIM or TRIMOPAN or METRONIDAZOLE or FLAGYL or METROLYL or PHENOXYMETHYLPENICILLIN or SULFAMETHOXAZOLE or OXACILLIN or CEPHALOTHIN or SULBACTAM or OFLOXACIN or CLINDAMYCIN or GENTAMYCIN or VANCOMYCIN).ab,ti. 32 (cyclosporin* or Chlortetracycline or Lymecycline or Methacycline or Rolitetracycline or lactam* or quinolone* or Carbapenem* or Thienamycins or cephalosporin* or cefamandole or Cefazolin or Cefonicid or Cefsulodin or Cephacetrile or Cephalexin or Cephaloridine or Cephamycin* or Monobactam* or Aztreonam or Moxalactam or Amdinocillin or Cyclacillin or Methicillin or Nafcillin or Oxacillin or Sulbactam).ab,ti. 33 (Nalidixic or Nedocromil or Oxolinic or Carteolol or Fluoroquinolones or Ciprofloxacin or Enoxacin or Norfloxacin or Ofloxacin or Pefloxacin or Cofactor).ab,ti. 34 (Amphotericin or Antimycin or Brefeldin or Bryostatin* or Candicidin or Epothilone* or Ketolide* or Roxithromycin or Filipin or Ivermectin or Josamycin or Leucomycins or Kitasamycin or Spiramycin or Lucensomycin or Maytansine or Mepartricin or Miocamycin or Natamycin or Nystatin or Oleandomycin or Troleandomycin or Oligomycin* or Rutamycin or Sirolimus or Tacrolimus or Tylosin).ab,ti. 35 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 36 23 and 35 |
| Ovid Embase | Trial registries (via CRS) |
| 1 exp sinusitis/ or paranasal sinus disease/ 2 atrophic rhinitis/ or chronic rhinitis/ or rhinosinusitis/ or vasomotor rhinitis/ 3 exp paranasal sinus/ 4 (rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis).tw. 5 (kartagener* adj3 syndrome*).tw. 6 (inflamm* adj5 sinus*).tw. 7 ((maxilla* or frontal*) adj3 sinus*).tw. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp chronic disease/ 10 exp recurrent disease/ 11 (chronic or persis* or recurrent*).tw. 12 9 or 10 or 11 13 8 and 12 14 CRSsNP.tw. 15 ((sinusitis or rhinitis) adj3 (chronic or persis* or recurrent*)).tw. 16 13 or 14 or 15 17 exp nose polyp/ 18 exp nose disease/ or exp nose/ 19 exp polyp/ 20 18 and 19 21 ((nose or nasal or rhino* or rhinitis or sinus* or sinonasal) adj3 (papilloma* or polyp*)).tw. 22 (rhinopolyp* or CRSwNP).tw. 23 16 or 17 or 20 or 21 or 22 24 exp antiinfective agent/ 25 exp antibiotic prophylaxis/ 26 exp lactam/ 27 exp quinolone derivative/ 28 exp macrolide/ 29 exp tetracycline derivative/ 30 (ANTIBIOT* or (ANTI adj3 BIOT*) or ANTIMICROBIAL* or (ANTI adj3 MICROBIAL*) or BACTERIOCID* or ANTIBACTERIAL* or (ANTI adj3 BACTERIAL*)).tw. 31 (PENICILLIN* or AMOXICILLIN or AMPICILLIN or CLAVULANIC or AMOXICLAV or AUGMENTIN or TICARCILLIN or TIMENTIN or FLUCLOXACILLIN or FLUAMPICIL or MAGNAPEN or PIPERACILLIN or TAZOCIN or CEPHALOSPORIN* or CEFACLOR or DISTACLOR or CEFADROXIL or BAXAN or CEFALEXIN or CEPOREX or KEFLEX or CEFAMANDOLE or KEFADOL or CEFAZOLIN* or KEFZOL or CEFIXIME or SUPRAX or CEFOTAXIME or CLAFORAN or CEFOXITIN or MEFOXIN or CEFPIROME or CEFROM or CEFPODOXIME or ORELOX or CEFPROZIL or CEFZIL or CEFRADINE or VELOSEL or CEFTAZIDIM or FORTUM or KEFADIM or CEFTRIAXONE or ROCEPHIN or CEFUROXIME or ZINACEF or ZINNAT or CEFONICID or AZTREONAM or AZACTAM or IMIPENEM or CILASTATIN or PRIMAXIN or MEROPENEM or TETRACYCLINE* or DETECLO or DEMECLEOCYCLIN or LEDERMYCIN or DOXYCYCLINE or VIBRAMYCIN or MINOCYCLINE or MINOCINE or OXYTETRACYCLINE or TERRAMYCIN or MACROLIDE* or ERYTHROMYCIN or ERYMAX or ERYTHROCIN or ERYTHROPED or AZITHROMYCIN or ZITHROMAX or CLARITHROMYCIN or KLARICID or TELITHROMYCIN or KETEK or TRIMOXAZOLE or SEPTRIN or TRIMETHOPRIM or MONOTRIM or TRIMOPAN or METRONIDAZOLE or FLAGYL or METROLYL or PHENOXYMETHYLPENICILLIN or SULFAMETHOXAZOLE or OXACILLIN or CEPHALOTHIN or SULBACTAM or OFLOXACIN or CLINDAMYCIN or GENTAMYCIN or VANCOMYCIN).tw. 32 (cyclosporin* or Chlortetracycline or Lymecycline or Methacycline or Rolitetracycline or lactam* or quinolone* or Carbapenem* or Thienamycins or cephalosporin* or cefamandole or Cefazolin or Cefonicid or Cefsulodin or Cephacetrile or Cephalexin or Cephaloridine or Cephamycin* or Monobactam* or Aztreonam or Moxalactam or Amdinocillin or Cyclacillin or Methicillin or Nafcillin or Oxacillin or Sulbactam).tw. 33 (Nalidixic or Nedocromil or Oxolinic or Carteolol or Fluoroquinolones or Ciprofloxacin or Enoxacin or Norfloxacin or Ofloxacin or Pefloxacin or Cofactor).tw. 34 (Amphotericin or Antimycin or Brefeldin or Bryostatin* or Candicidin or Epothilone* or Ketolide* or Roxithromycin or Filipin or Ivermectin or Josamycin or Leucomycins or Kitasamycin or Spiramycin or Lucensomycin or Maytansine or Mepartricin or Miocamycin or Natamycin or Nystatin or Oleandomycin or Troleandomycin or Oligomycin* or Rutamycin or Sirolimus or Tacrolimus or Tylosin).tw. 35 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 36 23 and 35 |
ClinicalTrials.gov Condition: rhinitis OR sinusitis OR rhinosinusitis OR (nose AND polyp*) OR (nasal AND polyp*) OR CRSsNP OR CRSwNP OR CRS ICTRP Title: rhinitis OR sinusitis OR rhinosinusitis OR CRSsNP OR CRSwNP OR CR OR All: (nose AND polyp*) OR (nasal AND polyp*) NB These searches were run from 1 March 2015 to 11 August 2015, when these terms were last searched to populate the Cochrane ENT trials register in CRS |
Appendix 2. Data extraction form
| REF ID: | Study title: |
| Date of extraction: | Extracted by: |
| General comments/notes (internal for discussion): |
| Flow chart of trial | ||
| Group A (Intervention) | Group B (Comparison) | |
| No. of people screened | ||
| No. of participants randomised ‐ all | ||
| No. randomised to each group | ||
| No. receiving treatment as allocated | ||
| No. not receiving treatment as allocated ‐ Reason 1 ‐ Reason 2 |
||
| No. dropped out (no follow‐up data for any outcome available) |
||
| No. excluded from analysis1 (for all outcomes) ‐ Reason 1 ‐ Reason 2 |
||
| 1This should be the people who received the treatment and were therefore not considered 'drop‐outs' but were excluded from all analyses (e.g. because the data could not be interpreted or the outcome was not recorded for some reason). | ||
| Information to go into 'Characteristics of included studies' table | |
| Methods | X arm, double/single/non‐blinded, [multicentre] parallel‐group/cross‐over/cluster‐RCT, with x duration of treatment and x duration of follow‐up |
| Participants |
Location: country, no of sites etc. Setting of recruitment and treatment: Sample size:
Participant (baseline) characteristics:
Inclusion criteria:[state diagnostic criteria used for CRS, polyps score if available] Exclusion criteria: |
| Interventions |
Intervention (n = x): drug name, method of administration, dose per day/frequency of administration, duration of treatment Comparator group (n = y): Use of additional interventions (common to both treatment arms): |
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
| Funding sources | 'No information provided'/'None declared'/State source of funding |
| Declarations of interest | 'No information provided'/'None declared'/State conflict |
| Notes | |
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Quote: "…" Comment: |
|
| Allocation concealment (selection bias) | Quote: "…" Comment: |
|
| Blinding of participants and personnel (performance bias) | Quote: "…" Comment: |
|
| Blinding of outcome assessment (detection bias) | Quote: "…" Comment: |
|
| Incomplete outcome data (attrition bias) | Quote: "…" Comment: |
|
| Selective reporting (reporting bias) | Quote: "…" Comment: |
|
| Other bias (see section 8.15) Insensitive/non‐validated instrument? |
Quote: "…" Comment: |
|
| Other bias (see section 8.15) | Quote: "…" Comment: |
| Findings of study: continuous outcomes | |||||||
| Results (continuous data table) | |||||||
| Outcome | Group A | Group B | Other summary stats/Notes | ||||
| Mean | SD | N | Mean | SD | N | Mean difference (95% CI), P values etc. | |
| Disease‐specific HRQL (instrument name/range) Time point: |
|||||||
| Generic HRQL (instrument name/range) Time point: |
|||||||
| Symptom score (overall) (instrument name/range) Time point: |
|||||||
|
Added total ‐ if scores reported separately for each symptom (range) Time point: |
|||||||
| Nasal blockage/obstruction/congestion (instrument name/range) |
|||||||
| Nasal discharge (instrument name/range) |
|||||||
| Facial pain/pressure (instrument name/range) |
|||||||
| Smell (reduction) (instrument name/range) |
|||||||
| Headache (instrument name/range) |
|||||||
| Cough (in children) (instrument name/range) |
|||||||
| Polyp size (instrument name/range) |
|||||||
| CT score (instrument name/range) |
|||||||
| Comments: | |||||||
| Results (dichotomous data table) | ||||||
| Outcome | Applicable review/intervention | Group A | Group B | Other summary stats/notes | ||
| No. of people with events | No. of people analysed | No. of people with events | No. of people analysed | P values, RR (95% CI), OR (95% CI) | ||
| Epistaxis/nose bleed | INCS Saline irrigation |
|||||
| Local irritation (sore throat, oral thrush, discomfort) | INCS Saline irrigation |
|||||
| Osteoporosis (minimum 6 months) | INCS | |||||
| Stunted growth (children, minimum 6 months) | INCS | Can also be measured as average height | ||||
| Mood disturbances | OCS | |||||
| Gastrointestinal disturbances (diarrhoea, nausea, vomiting, stomach irritation) |
OCS Antibiotics |
|||||
| Insomnia | OCS | |||||
| Osteoporosis (minimum 6 months) | INCS OCS |
|||||
| Discomfort | Saline irrigation | |||||
| Skin irritation | Antibiotics | |||||
| Anaphylaxis or other serious allergic reactions such as Stevens‐Johnson | Antibiotics | |||||
| Comments: | ||||||
Data and analyses
Comparison 1. Systemic antibiotics versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease‐specific health‐related quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Macrolides (at 3 months ‐ end of treatment) | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.98, ‐0.10] |
| 1.2 Macrolides (at 6 months ‐ 3 months after end of treatment) | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.81, 0.11] |
| 2 Gastrointestinal disturbances | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.22, 8.50] |
| 3 Suspected allergic reaction (rash or skin irritation) | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.67 [0.34, 128.86] |
| 4 Endoscopic score | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.85, 0.25] |
Comparison 2. Systemic antibiotics + saline + intranasal corticosteroids versus placebo + saline + intranasal corticosteroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease severity score (at 3 months ‐ end of treatment) | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.81, 2.79] |
| 2 Gastrointestinal disturbances | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.16, 7.10] |
Comparison 3. Systemic antibiotics versus intranasal corticosteroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease severity score (at 3 months ‐ end of treatment) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐2.11, 1.47] |
| 2 Individual symptom scores (at 3 months ‐ end of treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nasal obstruction | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [‐0.79, 1.47] |
| 2.2 Rhinorrhoea | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.81, 1.25] |
| 2.3 Facial pain | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.21, 0.11] |
| 2.4 Loss of sense of smell | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐1.75, 0.09] |
| 3 Endoscopic score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Combined swelling and discharge | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.78 [‐1.52, ‐0.04] |
| 3.2 Swelling | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.86, 0.08] |
| 3.3 Discharge | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.97, 0.19] |
Comparison 4. Systemic antibiotics versus oral steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gastrointestinal disturbances | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.14] |
| 2 Suspected allergic reaction (rash or skin irritation) | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.20, 19.62] |
| 3 Adverse events related to oral steroid use: insomnia | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.45] |
4.3. Analysis.
Comparison 4 Systemic antibiotics versus oral steroids, Outcome 3 Adverse events related to oral steroid use: insomnia.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Otten 1994.
| Methods | 2‐arm, double‐blind, parallel‐group RCT, with 1 week of treatment and follow‐up at 6 to 12 weeks | |
| Participants |
Location: the Netherlands Setting of recruitment and treatment: 4 different ENT practices Sample size: Number randomised: 79 patients Number completed: 75 patients: 37 in intervention, 38 in comparison Participant (baseline) characteristics:
Inclusion criteria: Purulent rhinitis of 3 months or more duration; pus shown in the middle nasal meatus by anterior rhinoscopy; sinus radiograph showing 1 of the following: a) bilateral complete opacity; b) bilateral mucosal swelling; c) unilateral mucosal swelling with unilateral opacity; d) unilateral mucosal swelling or unilateral opacity Exclusion criteria: Allergic to cephalosporin, anatomical lesion of the ear, nose and throat such as a severe septal deformation, a cleft palate or nasal polyps, previous treatment with antibiotics within 3 weeks of the start of this trial, and general contra‐indications such as cystic fibrosis or suffering from cardiac, renal, hepatic or other serious diseases |
|
| Interventions |
Intervention (n = 37): cefaclor at a dose of 20 mg/kg/day divided into 3 equal doses for 1 week Comparator group (n = 38): placebo for 1 week Use of additional interventions (common to both treatment arms): All participants received aspiration of sinus contents or antral washout (+ culturing of contents) and antroscopy prior to starting intervention Based on history, examination and sinus radiographs at 6 and 12 weeks, a sinus washout with antroscopy was carried out in participants who had persistent sinusitis at 6 or 12 weeks (or both) |
|
| Outcomes | No primary or secondary outcomes, as defined by the Cochrane review, were reported. Other outcomes reported by the study:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This was a randomised double‐blind study" Comment: no details on the method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No details on the method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "…placebo…", "double‐blind study" Comment: no further information on the placebo to assess blinding effectiveness |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "…placebo…", "double‐blind study" Comment: no information provided regarding whether the healthcare professionals completing the outcome measurements were blind to the treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient was withdrawn by his parents, one failed to have sinus radiographs, and two patients were withdrawn because a third consultation demonstrated a recurrent maxillary sinusitis that had initially resolved at the 6‐week consultation." Comment: 4/79 (5%) patients were not analysed, with reasons for exclusion stated, however it is not clear at what point the losses occurred |
| Selective reporting (reporting bias) | Unclear risk | The results are not well presented. No outcomes of interest relevant to the Cochrane review were reported in the paper. It is unclear whether adverse events were reported. We found no protocol for this study. |
| Other bias | Unclear risk | The paper did not provide a definition of "sinusitis resolved". |
Van Zele 2010.
| Methods | 3‐arm, double‐blind, multicentre, parallel‐group RCT, with 20 days duration of treatment and 12 weeks duration of follow‐up | |
| Participants |
Location: 5 sites in Belgium, Germany, Holland and Australia Setting of recruitment and treatment: not given Sample size: 47 Number randomised: 14 in antibiotics, 14 in oral steroids, 19 in placebo Number completed: 14 in antibiotics, 14 in oral steroids, 12 in placebo Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria: Participants must be at least 18 years with a diagnosis of bilateral nasal polyps at screening and baseline, which have recurred after surgical resection or nasal polyps that are grades 3 or 4 in both nares using the polyp scoring system. Women of childbearing potential must use a medically acceptable form of birth control as defined by the study. Male participants must agree to use an adequate form of birth control for the duration of the study as defined by the study. Participants with concurrent asthma must be maintained on no more than 1000 µg/d beclomethasone dipropionate or the equivalent. Nasal polyp score: 0 = no polyp; 1 = small polyps in the middle meatus not reaching below the inferior border of the middle concha; 2 = polyps reaching below the lower border of the middle turbinate; 3 = large polyps reaching the lower border of the inferior turbinate or polyps medial to the middle concha; 4 = large polyps causing almost complete congestion/obstruction of the inferior meatus Exclusion criteria: The following are the exclusion criteria of the study: pregnancy, breastfeeding or premenarcheal; oral corticosteroids within the 3 months before screening; systemic fungoid infections; known allergic reaction to methylprednisolone or tetracyclines; hypertension; diabetes (type 1 and 2); glaucoma; tuberculosis; herpes infection; zona ophthalmica; antineutrophil cytoplasmic antibodies such as Wegener's granulomatosis, Churg‐Strauss syndrome and microscopic polyangiitis. Participants with acute sinusitis or concurrent nasal infection or participants who have had a nasal or upper respiratory tract infection within 2 weeks of the screening visit; cystic fibrosis, primary ciliary dysfunction or Kartagener syndrome by history; those diagnosed with a parasitic infection; HIV‐positive or positive to hepatitis B surface antigen or C antibodies. Participants must not have had an acute asthmatic attack requiring admission to a hospital (excluding emergency department visits that resulted in direct discharge without hospitalisation) within the 4 weeks before screening. Participants must not have received immunotherapy within the previous 3 months. |
|
| Interventions |
Antibiotics (n = 14): doxycycline, oral, dose and frequency based on package information and evidence of tissue penetration Oral steroids (n = 14): oral methylprednisolone (32 mg/d on days 1 to 5; 16 mg/d on days 1 to 5; 8 mg/d on days 11 to 20) Placebo (n = 19): placebo, unlabelled lactose capsules, 20 days Use of additional interventions (common to all treatment arms): systemic or local corticosteroids or antibiotics were not allowed; if necessary nasal corticosteroids were permitted as rescue medication 2 months after dosing with the study medication |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
|
| Funding sources | "Supported by a grant from the Flemish Scientific Research Board, FWO Nr. A12/5‐HBKH 3 (holder of a Fundamenteel Klinisch Mandaat), by a postdoctoral grant from the Research Foundation Flanders (FWO), and by postdoctoral mandate from the Research Foundation Flanders (FWO)." | |
| Declarations of interest | "Disclosure of potential conflict of interest: P. J. Wormald has received royalties from Medtronic ENT, is a consultant for NeilMed, and has received research support from the Garnett Passe and Rodney Williams Foundation. W. Fokkens has received research support from GlaxoSmithKline and Stallergenes. A. Beule has received research support from the European Union. The rest of the authors have declared that they have no conflict of interest." | |
| Notes | The paper presented 2 comparisons of interest for this review: 1) Antibiotics (doxycycline) versus placebo 2) Antibiotics (doxycycline) versus short‐course oral steroids |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomly assigned to 3 groups by individuals not involved in the study." Comment: pg 1070, col 1, para 3. No information was provided about how the sequence was generated. The number of patients randomised was small and there is a risk the allocation between groups was not balanced (14 versus 19) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "… patients were randomly assigned to 3 groups by individuals not involved in the study" Comment: pg 1070, col 1, para 3 There is no information about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "…double‐blind…", "...Placebo (lactose) in unlabelled capsules" Comment: pg 1069, abstract: methods, pg 1070 methods Details of blinding are not clear within the paper and it does not detail whether the oral steroids and antibiotic medications were given on the same dosing schedule and were in an identical form. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Study participants and personnel were blind during the duration of the study. Randomisation codes were revealed to researchers after recruitment, data collection, and data entry" Comment: details of blinding are not clear within the paper and it is not clear whether the oral steroids and antibiotic medications were given on the same dosing schedule and were in an identical form, which could compromise blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 7/47 patients dropped out of the study (14.9%) but all were from the placebo group 7/19 (36.8%). This is an imbalance in drop‐out rate and the reasons for drop‐out include "unsatisfactory therapeutic effects", "withdrawal of consent" and "serious adverse events (asthma attack)". Patients who dropped out were still included in the analysis using the last observed carried forward. This may have had an effect on the overall results and no sensitivity analysis appears to have been completed to identify the impact. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes in the methods section were reported in the full paper, although many of them were presented graphically, without providing values at key time points. The data were not reported in a way that allowed inclusion in the meta‐analysis for the review. The protocol document was available (NCT00480298) and the outcomes appear to be consistent between the protocol and the paper. Adverse events were reported but no methods were provided for their collection. |
| Other bias | Unclear risk | Comment: details of the scales used to measure symptoms were not provided in the paper and there is no information on validation of any of the outcomes of interest to this review. There was an imbalance in the number of participants with "allergy" (oral steroids: 35.7%; placebo: 57.9%; antibiotics: 14.3%) and "aspirin intolerant" (oral steroids: 14.3%; placebo: 26.3%; antibiotics: 7.1%) in the baseline characteristics. There was not a statistical difference between the groups due to the study size being small. A sensitivity analysis was completed by the study authors to determine the number of aspirin‐intolerant patients affecting the results. |
Videler 2011.
| Methods | 2‐arm, double‐blind, multicentre, parallel‐group RCT, with 12 weeks duration of treatment and 24 weeks duration of follow‐up | |
| Participants |
Location: 6 centres: Amsterdam, Helsinki, Leuven, London, Tampere and Zagreb Setting of recruitment and treatment: tertiary referral centres Sample size: 60 Number randomised: 29 in azithromycin (AZM), 31 in placebo Number completed: 26 azithromycin (AZM), 27 in placebo Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (n = 29): oral azithromycin (AZM), 500 mg per day for 3 days, then 500 mg per week for 11 weeks Comparator group (n = 31): placebo with same amount, frequency and appearance as the AZM group Use of additional interventions: nasal saline irrigation twice daily (no details provided of the solution used) Intranasal or pulmonary steroids were allowed as long as the dosage was kept constant throughout study participation (a maximum of 2 times the regular dose was accepted (AZM: 19 (65.5%); placebo: 23 (64.5%)). |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
|
| Funding sources | "The azithromycin and placebo used in this study was kindly provided by PLIVA HRVATSKA d.o.o., Zagreb, Croatia." | |
| Declarations of interest | "None" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized, numbered study medication was kindly provided by pharmaceutical company. Study medication was allocated per centre in two randomised blocks, containing 6 packs of treatment each. Qualified subjects were given study medication with consecutive numbering" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomized, numbered study medication was kindly provided by pharmaceutical company. Study medication was allocated per centre in two randomised blocks, containing 6 packs of treatment each. Qualified subjects were given study medication with consecutive numbering" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind", "The placebo arm received the same amount of tablets, identical in appearance" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the outcomes were either patient‐reported or were assessed by the investigators who were blinded to the treatment allocation; assessed to have low risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/60 (15%) participants did not complete the study. Reasons for non‐completion are given and are distributed between the intervention and placebo group. No denominator given for each group at the final time point (telephone conference at 24 weeks). There were 2 participants who dropped out but the distribution between groups is not clear. In addition, the denominator for measuring adverse events (gastrointestinal disturbances) is not reported in the paper. |
| Selective reporting (reporting bias) | Unclear risk | Some items were not reported in full in the paper. Only partial data for the outcomes of SF‐36 and endoscopic score were presented, making presentation of numerical results difficult. The method for collecting adverse events was not presented in the paper. Protocol found: EUCTR‐2005‐001062‐14. The protocol did not mention the use of the patient response scale. The protocol states an aim to recruit 120 participants; 60 were actually recruited. |
| Other bias | Low risk | SNOT‐22 and SF‐36 are validated outcome measures. The validation of instruments used to measure symptom severity (0 to 10 VAS) and endoscopic scores was not clear from the paper. |
Wallwork 2006.
| Methods | 2‐arm, double‐blind, parallel‐group RCT, with a 12‐week duration of treatment and 24‐week duration of follow‐up | |
| Participants |
Location: Australia, 2 sites Setting of recruitment and treatment: hospital ENT department Sample size: 64 Number randomised: 29 in intervention, 35 in comparison Number completed: 26 in intervention, 30 in comparison Participant (baseline) characteristics: No specific information provided in the paper, other than that there was no difference between the 2 treatment groups for age and gender. No information or previous surgery.
Inclusion criteria: aged over 18 years. A CT scan was performed to confirm the diagnosis and was scored using the Lund‐Mackay CT scoring system (baseline scores not provided in the paper) Exclusion criteria: history of cystic fibrosis, primary ciliary dyskinesia, immune deficiency, allergic fungal sinusitis, nasal polyposis, and impairment of liver or renal function. Pregnant and breastfeeding women. Those taking medication with a known adverse interaction with macrolides or with a history of macrolide hypersensitivity. Used topical or systemic corticosteroids within 4 weeks of entering the study. |
|
| Interventions |
Intervention (n = 29): roxithromycin tablet, 150 mg daily for 3 months Comparator group (n = 35): placebo tablet daily for 3 months Use of additional interventions (common to both treatment arms): none listed |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes reported by the study:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…subjects were randomized by the pharmacy department, using a random number table…" Comment: pg 190, col 1, para 2 |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is no specific information about allocation concealment in the paper |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "…Patients and investigators were kept blinded to the randomisation until the completion of the study" Comment: pg 190, col 1, para 2 Placebo tablets were used in the control group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "…Patients and investigators were kept blinded to the randomisation until the completion of the study" Comment: pg 190, col 1, para 2 As the disease‐specific health‐related quality of life score (SNOT‐20) is patient‐reported it is expected that there will be a low risk of bias if patients did not know the group to which they were randomised. The endoscopy score was assessed by the primary author of the study and as the 'investigators' were blinded to treatment allocation, this is also assessed to be a low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 5/64 patients (7.8%) did not complete treatment. Reasons are provided in the paper. A further 3 patients did not complete the 12‐week post‐treatment follow‐up, which results in a total of 8/64 (12.5%). The paper reports that an intention‐to‐treat analysis was completed. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes as listed in the methods section were reported in the main text Adverse events do not appear to have been recorded No protocol could be found for this study |
| Other bias | Unclear risk | Information was provided about the validation of the SNOT‐20 instrument used to measure quality of life. No information was provided regarding the validation of the scale used to evaluate nasal polyps. There is no detailed information within the study with regards to the baseline characteristics of the treatment arms, apart from a statement that there were no differences between the 2 groups. |
Zeng 2011.
| Methods | 2‐arm, open‐label (non‐blinded), parallel‐group RCT, with a 12‐week duration of treatment and follow‐up | |
| Participants |
Location: Wuhan, China Setting of recruitment and treatment: otolaryngology department in teaching hospital Sample size: 43 Number randomised: 22 to clarithromycin, 21 to mometasone Number completed: 22 to clarithromycin, 21 to mometasone Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (n = 22): clarithromycin (Abbot China) 250 mg once daily for 12 weeks Comparator group (n = 21): mometasone furoate nasal spray (Schering‐Plough, China) 200 µg once daily for 12 weeks Use of additional interventions (common to both treatment arm): none listed |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
|
| Funding sources | Funded by the programme for New Century Excellent Talents in University from State Education Ministry (NCET‐07‐0326) and the National Nature Science Foundation of China (NSFC) grants 30872847 and 81020108018 to Z. Liu | |
| Declarations of interest | Quote: "The authors have no conflicts of interest to declare pertaining to this article" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomly assigned … using a random number table" |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is no information regarding allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "None of the patient withdrew from the study." |
| Selective reporting (reporting bias) | Unclear risk | All key outcomes appear to be well reported. No information regarding the measurement of adverse events was reported in the methods section and no results for adverse events are reported. No protocol could be found for this study. |
| Other bias | Unclear risk | Comment: although the endoscopic score was validated, there was no information about the validation of the assessment of symptom severity outcomes |
AZM: azithromycin CRS: chronic rhinosinusitis CRSsNP: chronic rhinosinusitis without nasal polyps CT: computerised tomography d: day ENT: ear, nose and throat EPOS: European Position Paper on Rhinosinusitis and Nasal Polyps ESS: endoscopic sinus surgery F: female M: male RCT: randomised controlled trial SEM: standard error of the mean SF‐36: quality of life scale SNOT‐20/‐22: Sinonasal Outcome Test‐20/‐22 VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agbim 1975 | DURATION: treatment and follow‐up only for 7 days |
| Amali 2015 | POPULATION: all patients underwent FESS at the start of the trial |
| Amini 2009 | DURATION: treatment duration was 3 weeks with follow‐up 8 weeks from the start of the trial |
| Ansari 2015 | DURATION: treatment time and follow‐up 4 weeks |
| Artigas 1989 | DURATION: treatment time and follow‐up 7 days |
| Beloborodova 1998 | DURATION: treatment time and follow‐up 10 days |
| Bezerra 2014 | STUDY DESIGN: not a randomised study |
| Bobacheva 2012 | POPULATION: all participants underwent surgery within 7 days of the start of the trial |
| Bonfils 2015 | DURATION: treatment time was 7 days with follow‐up 30 days from the start of the trial |
| Chatzimanolis 1998 | POPULATION: acute or recurrent sinusitis DURATION: treatment time and follow‐up was 10 to 12 days |
| Dellamonica 1994 | DURATION: treatment time was 10 days with follow‐up 30 days from the start of the trial |
| Desrosiers 2001 | DURATION: treatment time was 4 weeks with follow‐up 8 weeks from the start of the trial |
| Edelstein 1993 | POPULATION: half with acute sinusitis, half with "acute exacerbation of chronic sinusitis" DURATION: treatment time was 10 to 14 days with follow‐up 30 days from the start of the trial |
| El'kun 1999 | DURATION: treatment time and follow‐up for 7 days |
| Fan 2014 | DURATION: treatment time was 7 to 14 days with follow‐up for 4 weeks from the start of the trial |
| Hashiba 1997 | INTERVENTION: comparison of 2 antibiotics from the same class (clarithromycin and erythromycin) and therefore not an intra‐class comparison |
| Haxel 2015 | POPULATION: all patients had surgery within 2 weeks of the start of the trial |
| Hiratsuka 1996 | POPULATION: all participants had endoscopic sinus surgery at the start of the trial |
| Huck 1993 | DURATION: treatment time was 10 days with follow‐up for 18 days from the start of the trial |
| Husfeldt 1993 | DURATION: treatment time and follow‐up was for 7 to 14 days |
| IRCT201312299014N | INTERVENTION: phonophoresis of erythromycin versus pulsed ultrasound |
| Ishiura 1995 | POPULATION: sinobronchial syndrome |
| Jareoncharsri 2004 | DURATION: treatment time was 7 days with follow‐up of 21 days from the start of the trial |
| Jervis‐Bardy 2012 | DURATION: treatment time and follow‐up was for 28 days |
| Jiang 2008 | POPULATION: all patients underwent surgery at the start of the trial DURATION: treatment time and follow‐up was for 21 days |
| Kita 1995 | STUDY DESIGN: non‐randomised study |
| Korkmaz 2014 | DURATION: treatment time and follow‐up was for 8 weeks |
| Kunel'skaya 2008 | DURATION: treatment time and follow‐up was for 7 days |
| Legent 1994 | DURATION: treatment time was 9 days with follow‐up of 40 days from the start of the trial |
| Li 2000 | DURATION: follow‐up time too short |
| Li 2002 | DURATION: follow‐up time too short |
| Li 2014 | DURATION: treatment time and follow‐up was for 2 weeks |
| Mannhardt 1980 | DURATION: follow‐up in control arm was for 12 days |
| Namyslowski 1998 | DURATION: treatment time was 2 weeks with follow‐up of 6 weeks from the start of the trial |
| NCT01825408 | DURATION: study aimed to look at the duration of antibiotic treatment: 3 weeks versus 6 weeks treatment. The follow‐up period was 5 weeks and 8 weeks after the start of the trial. |
| NCT02307825 | POPULATION: all patients had surgery within 2 weeks of the start of the trial |
| Otten 1997 | INTERVENTION: study included 4 treatment arms, but none were comparisons of interest: (1) placebo; (2) xylometazoline (nasal decongestant) plus antibiotics; (3) drainage; (4) drainage plus xylometazoline plus antibiotics Same study as Otten 1990 |
| Peric 2011 | DURATION: treatment time and follow‐up was for 2 weeks |
| Portier 1996 | DURATION: treatment time and follow‐up was for 30 days |
| Rachelefsky 1982 | DURATION: treatment time and follow‐up was for 2 weeks |
| Schalek 2009 | POPULATION: all patients had surgery at the start of the trial |
| Sreenath 2015 | Comparison of short‐course (3 weeks) versus long‐course (6 weeks) antibiotics DURATION: patients were only followed up for 3 or 6 weeks |
| Sykes 1986 | DURATION: duration of treatment and follow‐up was 2 weeks |
| Varvianskaia 2013 | POPULATION: all patients had surgery at the start of the trial |
| Videler 2008 | DURATION: unclear follow‐up between 8 and 12 weeks |
| Watanabe 2003 | DURATION: treatment time and follow‐up in the control arm was for 4 weeks |
| Wei 2011 | DURATION: treatment time and follow‐up was for 7 weeks |
FESS: functional endoscopic sinus surgery
Characteristics of studies awaiting assessment [ordered by study ID]
Behm 2002.
| Methods | Unclear |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | "Health resource utilization: moxifloxacin compared to levofloxacin and amoxicillin clavulanate in reducing "practice time use" in the treatment of sinusitis" Awaiting receipt of paper |
Jiang 2001.
| Methods | Randomised controlled trial |
| Participants | Patients with chronic sinusitis due to undergo surgery |
| Interventions | Amoxicillin‐clavulanate potassium therapy versus no antibiotics |
| Outcomes | Bacteriology; it is unclear if any outcomes of interest were reported in the paper |
| Notes | It is unclear if outcomes were reported before surgery Awaiting receipt of full paper |
Kataoka 2003.
| Methods | Unclear |
| Participants | People with paranasal sinusitis complicating allergic rhinitis |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | Awaiting receipt of full paper |
Kim 2003.
| Methods | Unclear |
| Participants | Chronic sinusitis with nasal polyps and allergic rhinitis |
| Interventions | Roxithromycin 300 mg daily versus intranasal steroids |
| Outcomes | Patient symptoms and polyp size (no details on instruments used) |
| Notes | Written to study authors for more details about trial methods and results |
Ziuzio 1995.
| Methods | Unclear |
| Participants | Chronic purulent maxillary sinusitis |
| Interventions | Clindamycin versus clindamycin, gentamycin or amikacin with metronidazole versus others antibiotics |
| Outcomes | Unclear |
| Notes | Awaiting receipt of paper |
Characteristics of ongoing studies [ordered by study ID]
EUCTR 2005 (2005‐004736‐51).
| Trial name or title | 'Double‐blind randomized placebo‐controlled multinational, multicentre‐trial on prolonged macrolide treatment in patients with moderate/severe chronic rhinosinusitis' |
| Methods | Randomised controlled trial |
| Participants | People with moderate to severe chronic rhinosinusitis |
| Interventions | Macrolide antibiotics versus placebo |
| Outcomes | — |
| Starting date | 2006 |
| Contact information | Prof V Lund |
| Notes | Website says that the trial has completed. We contacted the study author for more information but no information was provided. |
Differences between protocol and review
As part of the discussions about the use of a total symptoms score we noted that many papers within the suite of reviews did not present information for all four elements of the EPOS criteria for defining chronic rhinosinusitis (EPOS 2012). In particular, many studies that only included patients with nasal polyps did not present information on facial pressure or pain. We made the decision that where individual symptoms were recorded, they should be presented within the outcome of disease severity symptom score within the paper as this information would be useful for the reader.
We changed the definition of a short course of antibiotics during the course of review. The original definition of a short course of antibiotics was 14 days. However, on a review of the evidence we found a study that gave antibiotics for 21 days. We felt that this was not the same type of information as from studies that gave antibiotics for three months and that the definition of short‐course antibiotics should be extended to one month as this was a more appropriate cut‐off point.
Contributions of authors
Lee Yee Chong: scoped, designed and wrote the protocol. For the review: abstract screening, full paper review, data extraction, data analysis, editing the report.
Karen Head: reviewed and edited the protocol. For the review: abstract screening, full paper review, data extraction, data analysis, drafted and editing the report.
Patorn Piromchai: for the review: abstract screening, full paper review, data extraction, clinical input into data analysis, drafting and editing the report.
Claire Hopkins: clinical guidance at all stages of project scoping and protocol development. For the review: clinical input into data analysis, reviewing and editing the report.
Carl Philpott: clinical guidance at all stages of project scoping and protocol development. For the review: clinical input into data analysis, reviewing and editing the report.
Anne GM Schilder: for the review: clinical input into data analysis, reviewing and editing the report.
Martin J Burton: helped to draft the protocol; clinical guidance at all stages of project scoping and protocol development. For the review: data extraction, clinical input into data analysis, reviewing and editing the report.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Funding to complete a suite of reviews on medical interventions for chronic rhinosinusitis in 2015/2016 (award reference 14/174/03), in addition to infrastructure funding for Cochrane ENT
Declarations of interest
Lee Yee Chong: none known.
Karen Head: none known.
Patorn Piromchai: none known.
Claire Hopkins: I have received financial support from several companies involved in producing instruments for sinus surgery: Acclarent, Sinusys, Cryolife and Medtronic.
Carl Philpott: I have previously received consultancy fees from the companies Acclarent, Navigant, Aerin Medical and Entellus.
Anne GM Schilder: Professor Anne Schilder is joint Co‐ordinating Editor of the Cochrane ENT Group, but had no role in the editorial process for this review. Her evidENT team at UCL is supported by her NIHR Research Professorship award with the remit to develop a UK infrastructure and programme of clinical research in ENT, Hearing and Balance. Her institution has received a grant from GSK for a study on the microbiology of acute tympanostomy tube otorrhoea.
Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of the Cochrane ENT Group, but had no role in the editorial process for this review.
New
References
References to studies included in this review
Otten 1994 {published data only}
- Otten HW, Antvelink JB, Ruyter de Wildt H, Rietema SJ, Siemelink RJ, Hordijk GJ. Is antibiotic treatment of chronic sinusitis effective in children?. Clinical Otolaryngology and Allied Sciences 1994;19(3):215‐7. [DOI] [PubMed] [Google Scholar]
Van Zele 2010 {published data only}
- Gevaert P, Zele T, Hellings P, Mayr S, Beule A, Ebbens F, et al. Doxycycline reduces ECP, MMP9 and nasal polyp size, in a double‐blind, randomized, placebo controlled, multicenter trial. World Allergy Organization Journal 2007;1 Suppl 3:S322. [Google Scholar]
- Zele T, Gevaert P, Holtappels G, Beule A, Wormald PJ, Mayr S, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. Journal of Allergy and Clinical Immunology 2010;125(5):1069‐76.e4. [DOI] [PubMed] [Google Scholar]
Videler 2011 {published data only}
- Videler WJ, Badia, L, Harvey RJ, Gane S, Georgalas C, Meulen FW, et al. Lack of efficacy of long‐term, low‐dose azithromycin in chronic rhinosinusitis: a randomized controlled trial. Allergy 2011;66:1457‐68. [DOI] [PubMed] [Google Scholar]
Wallwork 2006 {published data only}
- Wallwork B, Coman W, Mackay‐Sim A, Greiff L, Cervin A. A double‐blind, randomized, placebo‐controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope 2006;116(2):189‐93. [DOI] [PubMed] [Google Scholar]
Zeng 2011 {published data only}
- Zeng M, Long X‐B, Cui Y‐H, Liu Z. Comparison of efficacy of mometasone furoate versus clarithromycin in the treatment of chronic rhinosinusitis without nasal polyps in Chinese adults. American Journal of Rhinology & Allergy 2011;25(6):203‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agbim 1975 {published data only}
- Agbim OG. A comparison trial of doxycycline and ampicillin in the treatment of acute sinusitis. Chemotherapy 1975;21(Suppl 1):68‐75. [DOI] [PubMed] [Google Scholar]
Amali 2015 {published data only}
- Amali A, Saedi B, Rahavi‐Ezabadi S, Ghazavi H, Hassanpoor N. Long‐term postoperative azithromycin in patients with chronic rhinosinusitis: a randomized clinical trial. American Journal of Rhinology & Allergy 2015;29(6):421‐4. [DOI] [PubMed] [Google Scholar]
Amini 2009 {published data only}
- Amini M, Yarmohammadi M, Izadi P. A comparing study of clarithromycin XL with co‐amoxiclav for treatment of chronic sinusitis: a clinical trial. Iranian Journal of Clinical Infectious Diseases 2009;4(4):197‐201. [Google Scholar]
Ansari 2015 {published data only}
- Ansari NN, Naghdi S, Fathali M, Bartley J, Rastak MS. A randomized clinical trial comparing pulsed ultrasound and erythromycin phonophoresis in the treatment of patients with chronic rhinosinusitis. Physiotherapy Theory & Practice 2015;31(3):166‐72. [DOI] [PubMed] [Google Scholar]
Artigas 1989 {published data only}
- Artigas R, Iborra M, Ridao M, Ridao M. Study of the relative efficacy between amoxicillin and amoxicillin‐clavulanate in chronic sinusitis in pediatric age. Revista Espanola de Alergologia e Inmunologia Clinica 1989;4 Suppl 1:59. [Google Scholar]
Beloborodova 1998 {published data only}
- Beloborodova NV, Sorokin GV. Cost‐effect clinical and pharmacological efficacy of amoxicillin clavulanate (Amoxyclav) in pediatric otorhinolaryngology. Russian Bulletin of Perinatology and Pediatrics 1998;5(43):49‐56. [Google Scholar]
Bezerra 2014 {published data only}
- Bezerra T, Soter AC, Pezato R, Pinna F, Bachert C, Zele T, et al. Long‐term low‐dose doxycycline for difficult‐to‐treat chronic rhinosinusitis with polyps. Otolaryngology ‐ Head and Neck Surgery 2014;151(1S):121‐2. [Google Scholar]
Bobacheva 2012 {published data only}
- Bobacheva TI. The treatment of chronic polypous rhinosinusitis in the postoperative period with low doses of clarithromycin. Vestnik Otorinolaringologii 2012;4:68‐72. [PubMed] [Google Scholar]
Bonfils 2015 {published data only}
- Bonfils P, Escabasse V, Coste A, Gilain L, Louvrier C, Serrano E, et al. Efficacy of tobramycin aerosol in nasal polyposis. European Annals of Otorhinolaryngology, Head and Neck Diseases 2015;132(3):119‐23. [DOI] [PubMed] [Google Scholar]
Chatzimanolis 1998 {published data only}
- Chatzimanolis E, Marsan N, Lefatzis D, Pavlopoulos A. Comparison of roxithromycin with co‐amoxiclav in patients with sinusitis. Journal of Antimicrobial Chemotherapy 1998;41 Suppl B:81‐4. [DOI] [PubMed] [Google Scholar]
Dellamonica 1994 {published data only}
- Dellamonica P, Choutet P, Lejeune JM, Lucht F, Morgon A, Pessey JJ, et al. Efficacy and tolerance of cefotiam hexetil in the super‐infected chronic sinusitis. A randomized, double‐blind study in comparison with cefixime. Annales d Oto‐Laryngologie et de Chirurgie Cervico‐Faciale 1994;111(4):217‐22. [PubMed] [Google Scholar]
Desrosiers 2001 {published data only}
- Desrosiers MY, Salas‐Prato M. Treatment of chronic rhinosinusitis refractory to other treatments with topical antibiotic therapy delivered by means of a large‐particle nebulizer: results of a controlled trial. Otolaryngology ‐ Head and Heck Surgery 2001;125(3):265‐9. [DOI] [PubMed] [Google Scholar]
Edelstein 1993 {published data only}
- Edelstein DR, Avner SE, Chow JM, Duerksen RL, Johnson J, Ronis M, et al. Once‐a‐day therapy for sinusitis: a comparison study of cefixime and amoxicillin. Laryngoscope 1993;103(1 Pt 1):33‐41. [DOI] [PubMed] [Google Scholar]
El'kun 1999 {published data only}
- El'kun GB. Effectiveness of maxaquin and tarivid in combined therapy of patients with chronic sinusitis. Vestnik Otorinolaringologii 1999;6:45‐6. [PubMed] [Google Scholar]
Fan 2014 {published data only}
- Fan Y, Xu R, Hong H, Luo Q, Xia W, Ding M, et al. High and low doses of clarithromycin treatment are associated with different clinical efficacies and immunomodulatory properties in chronic rhinosinusitis. Journal of Laryngology & Otology 2014;128(3):236‐41. [DOI] [PubMed] [Google Scholar]
Hashiba 1997 {published data only}
- Hashiba M. Clinical effects of long‐term administration of macrolide antibiotics to patients with chronic paranasal sinusitis: results of past reports. Japanese Journal of Antibiotics 1997;50 Suppl A:5‐7. [PubMed] [Google Scholar]
Haxel 2015 {published data only}
- Haxel BR, Clemens M, Karaiskaki N, Dippold U, Kettern L, Mann WJ. Controlled trial for long‐term low‐dose erythromycin after sinus surgery for chronic rhinosinusitis. Laryngoscope 2015;125(5):1048‐55. [DOI] [PubMed] [Google Scholar]
Hiratsuka 1996 {published data only}
- Hiratsuka Y, Takagi A, Tokuda Y, Oishi N, Nagata H. Effect of roxithromycin on chronic sinusitis after endonasal sinus surgery. Oto‐Rhino‐Laryngology Tokyo 1996;39(2 Suppl 1):112‐6. [Google Scholar]
Huck 1993 {published data only}
- Huck W, Reed BD, Nielsen RW, Ferguson RT, Gray DW, Lund GK, et al. Cefaclor vs amoxicillin in the treatment of acute, recurrent, and chronic sinusitis. Archives of Family Medicine 1993;2(5):497‐503. [DOI] [PubMed] [Google Scholar]
Husfeldt 1993 {published data only}
- Husfeldt P, Egede F, Nielsen PB. Antibiotic treatment of sinusitis in general practice. A double‐blind study comparing ofloxacin and erythromycin. European Archives of Oto‐Rhino‐Laryngology 1993;250 Suppl 1:S23‐5. [DOI] [PubMed] [Google Scholar]
IRCT201312299014N {unpublished data only}
- IRCT201312299014N. Effect of topical vancomycin versus placebo on prevention of sinonasal polyposis recurrence after FESS (Functional Endoscopic Sinus Surgery). apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201312299014N20 (accessed 28 January 2016).
Ishiura 1995 {published data only}
- Ishiura Y, Fujimura M, Saito M, Shibata K, Nomura M, Nakatsumi Y, et al. Additive effect of continuous low‐dose ofloxacin on erythromycin therapy for sinobronchial syndrome. Respiratory Medicine 1995;89(10):677‐84. [DOI] [PubMed] [Google Scholar]
Jareoncharsri 2004 {published data only}
- Jareoncharsri P, Bunnag C, Fooanant S, Tunsuriyawong P, Voraprayoon S, Srifuengfung S, et al. An open label, randomized comparative study of levofloxacin and amoxicillin/clavulanic acid in the treatment of purulent sinusitis in adult Thai patients. Rhinology 2004;42(1):23‐9. [PubMed] [Google Scholar]
Jervis‐Bardy 2012 {published data only}
- Jervis‐Bardy J, Boase S, Psaltis A, Foreman A, Wormald P. A randomized trial of mupirocin sinonasal rinses versus saline in surgically recalcitrant staphylococcal chronic rhinosinusitis. Laryngoscope 2012;122(10):2148‐53. [DOI] [PubMed] [Google Scholar]
Jiang 2008 {published data only}
- Jiang R‐S, Liang K‐L, Yang K‐Y, Shiao J‐Y, Su M‐C, Hsin C‐H, et al. Postoperative antibiotic care after functional endoscopic sinus surgery. American Journal of Rhinology 2008;22(6):608‐12. [DOI] [PubMed] [Google Scholar]
Kita 1995 {published data only}
- Kita H, Takezawa H, Isobe M, Kataura A. Long‐term low dose erythromycin (EM) and roxithromycin (RXM) therapy for chronic sinusitis. Practica Otologica, Supplement 1995;84:62‐9. [Google Scholar]
Korkmaz 2014 {published data only}
- Korkmaz H, Ocal B, Tatar EC, Tatar I, Ozdek A, Saylam G, et al. Biofilms in chronic rhinosinusitis with polyps: is eradication possible?. European Archives of Oto‐rhino‐laryngology 2014;271(10):2695‐702. [DOI] [PubMed] [Google Scholar]
Kunel'skaya 2008 {published data only}
- Kunel'skaya NL, Gurov AV, Kudriavtseva IS, Kafarskaia LI, Izotova GN. Study of the efficacy of cefixime (suprax) in patients with acute and recurrent chronic purulent sinusitis. Vestnik Otorinolaringologii 2008;6:55‐8. [PubMed] [Google Scholar]
Legent 1994 {published data only}
- Legent F, Bordure P, Beauvillain C. Comparative study of ciprofloxacin and amoxicillin‐clavulanic acid in the treatment of chronic sinusitis [Etude comparative de la ciprofloxacine et de l'amoxicilline/acide clavulanique dans le traitement des sinusites chroniques]. Medecine et Maladies Infectieuses 1993;23(6):8‐13. [Google Scholar]
- Legent F, Bordure P, Beauvillain C, Berche P. A double‐blind comparison of ciprofloxacin and amoxycillin/clavulanic acid in the treatment of chronic sinusitis. Chemotherapy 1994;40 Suppl 1:8‐15. [DOI] [PubMed] [Google Scholar]
Li 2000 {published data only}
- Li XP, Qin ZM, Zheng RH, Tan QL, Zhou YY, Zhu L, et al. Comparison of the effectiveness of levofloxacin and cefuroxime for the treatment of sinusitis. Lin Chuang Erh Pi Yen Hou Ko Tsa Chih [Journal of Clinical Otorhinolaryngology] 2000;14(12):573‐5. [PubMed] [Google Scholar]
Li 2002 {published data only}
- Li ZY, Liang YL. Comparison of effect of metronidazole combined with lincomycin and gentamycin combined with dexamethasone in treatment of chronic maxillary sinusitis. Zhongguo Xin Yao Yu Lin Chuang Za Zhi [Chinese Journal of New Drugs and Clinical Remedies] 2002;1:28‐9. [Google Scholar]
Li 2014 {published data only}
- Li Y, Wu T. A randomized controlled study of treating chronic rhinosinusitis with macrolides. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi [Journal of Clinical Otorhinolaryngology, Head, and Neck Surgery] 2014;28(17):1289‐91. [PubMed] [Google Scholar]
Mannhardt 1980 {published data only}
- Mannhardt J, Held J. Randomized comparative study of the therapeutic action of amoxicillin and doxycycline in sinusitis. Die Therapiewoche 1980;30(12):2087‐9. [Google Scholar]
Namyslowski 1998 {published data only}
- Namyslowski G, Misiolek M, Czecior E, Malafiej E, Orecka B, Namyslowski P, et al. Comparison of the efficacy and tolerability of amoxycillin/clavulanic acid 875 mg b.i.d. with cefuroxime 500 mg b.i.d. in the treatment of chronic and acute exacerbation of chronic sinusitis in adults. Journal of Chemotherapy (Florence, Italy) 2002;14(5):508‐17. [DOI] [PubMed] [Google Scholar]
- Namyslowski G, Misiolek M, Malafiej E, Czecior E, Orecka B, Woch G, et al. Randomized clinical trial comparing the efficacy and safety of Augmentin versus cefuroxime in the treatment of chronic sinusitis in adult patients. Medical Science Monitor 1998;4(3):551‐4. [Google Scholar]
NCT01825408 {unpublished data only}
- NCT01825408. Duration of antibiotic therapy as part of maximal medical therapy for chronic rhinosinusitis. clinicaltrials.gov/show/NCT01825408 (accessed 28 January 2016).
NCT02307825 {unpublished data only}
- NCT02307825. Azithromycin for patients with chronic rhinosinusitis failing medical and surgical therapy (AZI‐CRS). https://clinicaltrials.gov/show/NCT02307825 (accessed 28 January 2016).
Otten 1997 {published data only}
- Otten FW, Grote JJ. Otitis media with effusion and chronic upper respiratory tract infection in children: a randomized, placebo‐controlled clinical study. Laryngoscope 1990;100(6):627‐33. [DOI] [PubMed] [Google Scholar]
- Otten FWA. Conservative treatment of chronic maxillary sinusitis in children. Long‐term follow‐up. Acta Oto‐rhino‐laryngologica Belgica 1997;51(3):173‐5. [PubMed] [Google Scholar]
- Otten FWA, Grote JJ. Treatment of chronic maxillary sinusitis in children. International Journal of Pediatric Otorhinolaryngology 1988;15(3):269‐78. [DOI] [PubMed] [Google Scholar]
Peric 2011 {published data only}
- Peric A, Vojvodic D, Vukomanovic‐Durdevic B. Nasal polyps with more intense epithelial eosinophil infiltration respond worse to long‐term low‐dose treatment by clarithromycin. Advances in Clinical and Experimental Medicine 2011;20(3):325‐34. [Google Scholar]
Portier 1996 {published data only}
- Portier H, Bonfils P, Chobaut JC, Lallemant JG, Martin C, Pessey JJ, et al. Clinical efficacy and safety of cefotiam hexetil for over infected chronic sinusitis. Comparison with amoxicillin/clavulanic acid. [French]. Medecine et Maladies Infectieuses 1996;26(12):1188‐93. [Google Scholar]
Rachelefsky 1982 {published data only}
- Rachelefsky GS, Katz RM, Siegel SC. Chronic sinusitis in children with respiratory allergy: the role of antimicrobials. Journal of Allergy and Clinical Immunology 1982;69(4):382‐7. [DOI] [PubMed] [Google Scholar]
Schalek 2009 {published data only}
- Schalek P, Petrás P, Klement V, Hahn A. Short‐term antibiotics treatment in patients with nasal polyps and enterotoxins producing Staphylococcus aureus strains. European Archives of Oto‐rhino‐laryngology 2009;266(12):1909‐13. [DOI] [PubMed] [Google Scholar]
Sreenath 2015 {published data only}
- Sreenath SB, Taylor RJ, Miller JD, Ambrose EC, Rawal RB, Ebert CS, et al. A prospective randomized cohort study evaluating 3 weeks vs 6 weeks of oral antibiotic treatment in the setting of "maximal medical therapy" for chronic rhinosinusitis. International Forum of Allergy and Rhinology 2015;5(9):820‐8. [DOI] [PubMed] [Google Scholar]
Sykes 1986 {published data only}
- Sykes DA, Wilson R, Chan KL, Mackay IS, Cole PJ. Relative importance of antibiotic and improved clearance in topical treatment of chronic mucopurulent rhinosinusitis. A controlled study. Lancet 1986;2(8503):359. [DOI] [PubMed] [Google Scholar]
Varvianskaia 2013 {published data only}
- Varvianskaia AV, Lopatin AS. The effectiveness of long‐term treatment of polypous rhinosinusitis with low doses of macrolides. Vestnik Otorinolaringologii 2013;5:22‐7. [PubMed] [Google Scholar]
Videler 2008 {published data only}
- Videler WJM, Drunen CM, Reitsma JB, Fokkens W J. Nebulized bacitracin/colimycin: a treatment option in recalcitrant chronic rhinosinusitis with Staphylococcus aureus? A double‐blind, randomized, placebo‐controlled, cross‐over pilot study. Rhinology 2008;46(2):92‐8. [PubMed] [Google Scholar]
Watanabe 2003 {published data only}
- Watanabe T, Ichinomiya K, Suzuki M. Combined therapy for patients with chronic paranasal sinusitis using a macrolide antibiotic and YAMIK catheter. Japanese Journal of Antibiotics 2003;56 Suppl A:154‐7. [PubMed] [Google Scholar]
Wei 2011 {published data only}
- NCT00465530. Comparing the use of saline or saline plus gentamycin in nasal irrigation to treat chronic sinusitis in children. https://clinicaltrials.gov/show/NCT00465530 (accessed 28 January 2016).
- Wei JL, Sykes KJ, Johnson P, He J, Mayo MS. Safety and efficacy of once‐daily nasal irrigation for the treatment of pediatric chronic rhinosinusitis. Laryngoscope 2011;121(9):1989‐2000. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Behm 2002 {published data only}
- Behm J, Corcoran G. Health resource utilization: moxifloxacin compared to levofloxacin and amoxicillin clavulanate in reducing "practice time use" in the treatment of sinusitis. American Journal of Respiratory and Critical Care Medicine 2002;165(8 Suppl):A107. [Google Scholar]
Jiang 2001 {published data only}
- Jiang RS, Wang JW. Bacteriology of chronic sinusitis after amoxicillin‐clavulanate potassium therapy. Otolaryngology ‐ Head and Neck Surgery 2001;124(6):683‐6. [DOI] [PubMed] [Google Scholar]
Kataoka 2003 {published data only}
- Kataoka S, Ogasawara K, Ishimitsu R, Tagami H, Iwamoto J, Kawauchi H. Combined drug therapy for patients with intractable paranasal sinusitis complicating allergic rhinitis. Japanese Journal of Antibiotics 2003;56 Suppl A:158‐61. [PubMed] [Google Scholar]
Kim 2003 {published data only}
- Kim BG, Lee D‐M, Jo J‐H, Jung D‐G, Kang J‐M, Kim S‐W. Effect of roxithromycin and intranasal fluticasone spray in reducing symptoms of chronic sinusitis, polyp size and IL‐4 in allergic patients. Journal of Rhinology 2003;10(1):37‐41. [Google Scholar]
Ziuzio 1995 {published data only}
- Ziuzio S, Sakson B. Results of conservative treatment of chronic purulent sinusitis. Otolaryngologia Polska 1995;49 Suppl 23:178‐82. [PubMed] [Google Scholar]
References to ongoing studies
EUCTR 2005 (2005‐004736‐51) {published data only}
- EUCTR 2005 (2005‐004736‐51). Double‐blind randomized placebo‐controlled multinational, multicentre‐trial on prolonged macrolide treatment in patients with moderate/severe chronic rhinosinusitis. clinicaltrialsregister.eu/ctr‐search/trial/2005‐004736‐51 accessed 28 January 2016.
Additional references
Balk 2012
- Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ. Empirical assessment of within‐arm correlation imputation in trials of continuous outcomes [Internet]. Report No.: 12(13)‐EHC141‐EF. AHRQ Methods for Effective Health Care. Rockville (MD): Agency for Healthcare Research and Quality, 2012. [PubMed] [Google Scholar]
Bewick 2014
- Bewick JC, Egro FM, Masterson LM, Philpott CM. Preliminary findings: the feasibility study for a randomized controlled trial of clarithromycin in chronic rhinosinusitis. Otolaryngology ‐ Head and Neck Surgery 2014;15(Suppl 1):P25. [Google Scholar]
Bucher 2004
- Bucher HC, Tschudi P, Young J, Périat P, Welge‐Lüussen A, Züst H, et al. Effect of amoxicillin‐clavulanate in clinically diagnosed acute rhinosinusitis: a placebo‐controlled, double‐blind, randomized trial in general practice. Archives of Internal Medicine 2004;163(15):1793‐8. [DOI] [PubMed] [Google Scholar]
Cho 2012
- Cho SH, Hong SJ, Han B, Lee SH, Suh L, Norton J, et al. Age‐related differences in the pathogenesis of chronic rhinosinusitis. Journal of Allergy and Clinical Immunology 2012;129(3):858‐60.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2016a
- Chong LY, Head K, Hopkins C, Philpott C, Burton MJ, Schilder AGM. Different types of intranasal steroids for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011993.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2016b
- Chong LY, Head K, Hopkins C, Philpott C, Schilder AGM, Burton MJ. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011996.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2016c
- Chong LY, Head K, Hopkins C, Philpott C, Glew S, Scadding G, et al. Saline irrigation for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011995.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988. [Google Scholar]
DeMarcantonio 2011
- DeMarcantonio MA, Han JK. Nasal polyps: pathogenesis and treatment implications. Otolaryngologic Clinics of North America 2011;44(3):685‐95, ix. [DOI] [PubMed] [Google Scholar]
Donath 2013
- Donath E, Chaudhry A, Hernandez‐Aya LF, Lit L. A meta‐analysis on the prophylactic use of macrolide antibiotics for the prevention of disease exacerbations in patients with chronic obstructive pulmonary disease. Respiratory Medicine 2013;107:1385‐92. [DOI] [PubMed] [Google Scholar]
Ebbens 2010
- Ebbens FA, Toppila‐Salmi SK, Renkonen JA, Renkonen RL, Mullol J, Drunen CM, et al. Endothelial L‐selectin ligand expression in nasal polyps. Allergy 2010;65(1):95‐102. [DOI] [PubMed] [Google Scholar]
Ebbens 2011
- Ebbens FA, Toppila‐Salmi S, Groot EJ, Renkonen J, Renkonen R, Drunen CM, et al. Predictors of post‐operative response to treatment: a double blind placebo controlled study in chronic rhinosinusitis patients. Rhinology 2011;49(4):413‐9. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOS 2007
- Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps. Rhinology. Supplement 2007;20:1‐136. [PubMed] [Google Scholar]
EPOS 2012
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology. Supplement 2012;50 Suppl 23:1‐298. [PubMed] [Google Scholar]
Fokkens 2007
- Fokkens W, Lund V, Mullol J. European Position Paper on Rhinosinusitis and Nasal Polyps 2007. Rhinology 2007;45(Suppl 20):1‐139. [PubMed] [Google Scholar]
Genoway 2011
- Genoway KA, Philpott CM, Javer AR. Pathogen yield and antimicrobial resistance patterns of chronic rhinosinusitis patients presenting to a tertiary rhinology centre. Journal of Otolaryngology ‐ Head & Neck Surgery 2011;40(3):232‐7. [PubMed] [Google Scholar]
Gliklich 1995
- Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngology ‐ Head and Neck Surgery 1995;113(1):104‐9. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Haruna 2009
- Haruna S, Shimada C, Ozawa M, Fukami S, Moriyama H. A study of poor responders for long‐term, low‐dose macrolide administration for chronic sinusitis. Rhinology 2009;47(1):66‐71. [PubMed] [Google Scholar]
Harvey 2009
- Harvey RJ, Wallwork BD, Lund VJ. Anti‐inflammatory effects of macrolides: applications in chronic rhinosinusitis. Immunology and Allergy Clinics of North America 2009;29(4):689‐703. [DOI] [PubMed] [Google Scholar]
Hastan 2011
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe ‐ an underestimated disease. A GA2LEN study. Allergy 2011;66(9):1216‐23. [DOI] [PubMed] [Google Scholar]
Hatipoglu 2005
- Hatipoglu U, Rubinstein I. Treatment of chronic rhinosinusitis with low‐dose, long‐term macrolide antibiotics: an evolving paradigm. Current Allergy and Asthma Reports 2005;5:491‐4. [DOI] [PubMed] [Google Scholar]
Head 2016a
- Head K, Chong LY, Hopkins C, Philpott C, Burton MJ, Schilder AGM. Short‐course oral steroids alone for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011991.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Head 2016b
- Head K, Chong LY, Hopkins C, Philpott C, Schilder AGM, Burton MJ. Short‐course oral steroids as an adjunct therapy for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011992.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopkins 2009
- Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22‐item Sinonasal Outcome Test. Clinical Otolaryngology 2009;34(5):447‐54. [DOI] [PubMed] [Google Scholar]
Hopkins 2016
- Hopkins C, Philpott C, Crowe S, Regan S, Degun A, Papachristou I, et al. Identifying the most important outcomes for systematic reviews of interventions for rhinosinusitis in adults: working with Patients, Public and Practitioners. Rhinology 2016; Vol. 54, issue 1:20‐6. [DOI: 10.4193/Rhin15.199] [DOI] [PubMed]
Inamura 2000
- Inamura K, Ohta N, Fukase S, Kasajima N, Aoyagi M. The effect of erythromycin on human peripheral neutrophil apoptosis. Rhinology 2000;38:124‐9. [PubMed] [Google Scholar]
Iyer 2016
- Iyer G, Alexander GC. Cardiovascular risks associated with clarithromycin. BMJ 2016;352:i23. [DOI: 10.1136/bmj.i23] [DOI] [PubMed] [Google Scholar]
Jaeschke 1989
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials 1989;10(4):407‐15. [PUBMED: 2691207] [DOI] [PubMed] [Google Scholar]
Kern 2008
- Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi‐Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. American Journal of Rhinology 2008;22(6):549‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keswani 2012
- Keswani A, Chustz RT, Suh L, Carter R, Peters AT, Tan BK, et al. Differential expression of interleukin‐32 in chronic rhinosinusitis with and without nasal polyps. Allergy 2012;67(1):25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lanza 1997
- Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngology ‐ Head and Neck Surgery 1997;117:S1‐7. [DOI] [PubMed] [Google Scholar]
Larsen 2004
- Larsen P, Tos M. Origin of nasal polyps: an endoscopic autopsy study. Laryngoscope 2004;114(4):710‐9. [DOI] [PubMed] [Google Scholar]
MacLaughlin 2000
- MacLaughlin EJ, Saseen JJ, Malone DC. Costs of beta‐lactam allergies: selection and costs of antibiotics for patients with a reported beta‐lactam allergy. Archives of Family Medicine 2000;9(8):722‐6. [DOI] [PubMed] [Google Scholar]
Miyanohara 2000
- Miyanohara T, Ushikai M, Matsune S, Ueno K, Katahira S, Kurono Y. Effects of clarithromycin on cultured human nasal epithelial cells and fibroblasts. Laryngoscope 2000;110:126‐31. [DOI] [PubMed] [Google Scholar]
Piromchai 2011
- Piromchai P, Thanaviratananich S, Laopaiboon M. Systemic antibiotics for chronic rhinosinusitis without nasal polyps in adults. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD008233.pub2] [DOI] [PubMed] [Google Scholar]
Pynnonen 2013
- Pynnonen MA, Venkatraman G, Davis G. Macrolide therapy for chronic rhinosinusitis: a meta‐analysis. Otolaryngology ‐ Head and Neck Surgery 2013;148(3):366‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ragab 2004
- Ragab SM, Lund VJ, Scadding G. Evaluation of the medical and surgical treatment of chronic rhinosinusitis: a prospective, randomised, controlled trial. Laryngoscope 2004;114(5):923‐30. [DOI] [PubMed] [Google Scholar]
Ragab 2010
- Ragab SM, Lund VJ, Scadding G, Saleh HA, Khalifa MA. Impact of chronic rhinosinusitis therapy on quality of life: a prospective randomized controlled trial. Rhinology 2010;48(3):305‐11. [DOI] [PubMed] [Google Scholar]
Revicki 2008
- Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient‐reported outcomes. Journal of Clinical Epidemiology 2008;61(2):102‐9. [PUBMED: 18177782] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shi 2014
- Shi ZL, Peng H, Hu XW, Hu JG. Effectiveness and safety of macrolides in bronchiectasis patients: a meta‐analysis and systematic review. Pulmonary Pharmacology and Therapeutics 2014;28(2):171‐8. [DOI] [PubMed] [Google Scholar]
Soler 2010
- Soler ZM, Smith TL. Quality‐of‐life outcomes after endoscopic sinus surgery: how long is long enough?. Otolaryngology ‐ Head and Neck Surgery 2010;143(5):621‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tamaoki 2004
- Tamaoki J, Kadota J, Takizawa H. Clinical implications of the immunomodulatory effects of macrolides. American Journal of Medicine 2004;117:5S‐11S. [DOI] [PubMed] [Google Scholar]
Tan 2011
- Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology 2011;128(6):1198‐206.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomassen 2011
- Tomassen P, Zele T, Zhang N, Perez‐ Novo C, Bruaene N, Gevaert P, et al. Pathophysiology of chronic rhinosinusitis. Proceedings of the American Thoracic Society 2011;8(1):115‐20. [DOI] [PubMed] [Google Scholar]
van Drunen 2009
- Drunen CM, Reinartz SM, Wigman J, Fokkens W. Inflammation in chronic rhinosinusitis and nasal polyposis. Immunology and Allergy Clinics of North America 2009;29(4):621‐9. [DOI] [PubMed] [Google Scholar]
Wozniak 2004
- Wozniak DJ, Keyser R. Effects of subinhibitory concentrations of macrolide antibiotics on Pseudomonas aeruginosa. Chest 2004;125:62S‐9S. [DOI] [PubMed] [Google Scholar]
Zhang 2008
- Zhang N, Zele T, Perez‐Novo C, Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T‐effector cells orchestrate mucosal inflammation in chronic sinus disease. Journal of Allergy and Clinical Immunology 2008;122(5):961‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2009
- Zhang XH, Lu X, Long XB, You XJ, Gao QX, Cui YH, et al. Chronic rhinosinusitis with and without nasal polyps is associated with decreased expression of glucocorticoid‐induced leucine zipper. Clinical and Experimental Allergy 2009;39(5):647‐54. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chong 2015
- Chong LY, Head K, Hopkins C, Philpott C, Burton MJ. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD011994] [DOI] [PMC free article] [PubMed] [Google Scholar]



