Abstract
Parkinson’s disease (PD) is a neurological disorder that affects the movement of the human body. It is primarily characterized by reduced dopamine levels in the brain. The causative agent of PD is still unclear but it is generally accepted that α-synuclein has a central role to play. It is also known that gap-junctions and associated connexins are complicated structures that play critical roles in nervous system signaling and associated misfunctioning. Thus, our current article emphasizes how, alongside α-synuclein, ion-channels, gap-junctions, and related connexins, all play vital roles in influencing multiple metabolic activities of the brain during PD. It also highlights that ion-channel and gap-junction disruptions, which are primarily mediated by their structural-functional changes and alterations, have a role in PD. Furthermore, we discussed available drugs and advanced therapeutic interventions that target Parkinson’s pathogenesis. In conclusion, it warrants creating better treatments for PD patients. Although, dopaminergic replenishment therapy is useful in treating neurological problems, such therapies are, however, unable to control the degeneration that underpins the disease, thereby declining their overall efficacy. This creates an additional challenge and an untapped scope for neurologists to adopt treatments for PD by targeting the ion-channels and gap-junctions, which is well-reviewed in the present article.
Subject terms: Ion channels in the nervous system, Parkinson's disease
Introduction
Parkinson’s disease (PD) is a progressing neurological ailment that causes mortality and impacts 1–3% of the worldwide community around the age of 60 years1. There are two main types of PD: hereditary, which is biologically acquired either in an autosomal dominant or recessive way, and sporadic (idiopathic), which is thought to emerge through genomic and environmental interactions2. Genetic PD accounts for 10–15% of overall occurrences, while all others are categorized as sporadic3. There are seven known causative alleles for familial PD: α-synuclein (SNCA), glucocerebrosidase (GBA), leucine-rich repeat kinase 2 (LRRK2), vacuolar protein sorting-associated protein 35 (VPS35), parkin RBR E3 ubiquitin-protein ligase (PARK2), phosphatase, and tensing homolog-induced kinase 1 (PARK7)4,5. PD is pathologically characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) accompanied by Lewy bodies, i.e. inclusion bodies rich in α-synuclein6. In addition to other mechanisms, α-synuclein also forms an amyloidogenic, helix structure that interacts with membrane lipids of the brain and this interaction can be exploited further for the treatment of PD. The pathological hallmarks of PD are gait freezing, tremor, rigidity, bradykinesia, and postural instability, out of which a known classical triad is tremor, bradykinesia/akinesia, and rigidity/postural instability7. Studies on the central nervous system (CNS) have shown that a hundred billion nerve cells8 are interconnected and communicate via synapses and thus mediate complicated signaling networks among themselves9. Within this complex neural network, gap-junction-based synaptic vesicles remain scattered, thereby playing an important function in the developmental processes of the CNS. Gap-junctions consist of connexins (Cxs) in vertebrates and innexins in invertebrate organisms. Connexins are a broad group of membrane-spanning peptides which facilitate cell-cell interaction as well as the exchange of ions and tiny signaling chemicals9. These gap-junctions act as a connection between the nerve cells and glia, namely astrocytes, microglia, and oligodendrocytes. Therefore, many structural or functional alterations in these junctions may lead to PD pathogenesis10.
The blood-brain barrier (BBB) remains intact during CNS diseases like PD11. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-model of PD exhibited an increase of a specific Cx expression in the striatum, although the coupling of astrocytes was not increased12,13. The alteration of gap-junctions in the brains of PD patients has not yet been reported. Therefore, the pathological role of gap-junctions in PD is still ambiguous. Although most of the mechanisms of tremors and dyskinesias which are commonly observed in PD patients are still obscure, the inferior olive has been focused on as the pathological generator of tremors14,15. Neurons of the inferior olive are electrically coupled through gap-junctions, which play a role in creating oscillatory activity. Some studies reported that the Gap-Junction Intercellular Communication (GJIC) of inferior olive neurons is responsible for tremors. However, no difference in the severity of tremor induced by harmaline was observed between Cx36 knockout (KO) mice and wild-type mice13,14,16. Neurons, astrocytes, oligodendrocytes, and microglial cells express a diverse set of Cxs which are required during intercellular interaction and the transport of small molecules, ions, as well as neuronal and glial transmitters17. They not only play an important function in the CNS but also play a pathological role in many neurodegenerative diseases like PD, Alzheimer’s Disease (AD), and Huntington’s Disease (HD). Many Cxs are substantially upregulated in PD, while a few are negatively regulated. In PD, increased production of some Cxs in the cerebral endothelial cells has been linked to capillary leaking and improved endothelial cell interaction. Similarly, most ion-channels control their firing action potential and the pivot-like activity of dopamine, released from the axonal terminus of subcortical basal ganglia, functions like a motor18. For example, positively charged potassium is responsible for maintaining balance and equilibrium within the cell thereby maintaining its volume and at the same time maintaining the potential of the cellular membrane18. All of these play a major role in the spreading of PD. Therefore, the current article reviews a diverse array of gap-junctions and ion-channels responsible for PD and their mechanistic overview to address the need for a better understanding of the cause of PD pathophysiology.
Among environmental factors, several occupations such as agriculture, working with pesticides, and heavy metals have higher risks of PD. Studies conducted on highly populated urban areas have also found significant correlations between industrial airborne heavy metal pollution and ambient air pollution from traffic, and an augmented chance of PD onset10. Other investigations have associated rural residency as a causative factor for idiopathic PD3. Individual genomes are all distinct, and people exposed to the same environmental factor are sometimes impacted differentially, generating a wide range of illnesses including PD3. Both the combination impact of hereditary as well as socio-ecological variables might affect the development of individual illness by compositionally changing DNA3. Caffeine, for instance, is an adenosine A2A (ADORA2A gene) receptor antagonist that promotes dopaminergic neurotransmitter release19, and ADORA2A polymorphism has been reported to lessen the incidence of PD19. Pesticide compounds and toxic heavy elements, on the other hand, have a detrimental impact and exacerbate PD by producing gene variants connected to genetic PD (e.g. PARK1, LRRK2 (Leucine-rich repeat kinase 2), PINK1 (PTEN-induced kinase 1)), which culminate in PD-associated processes such as mitochondrial malfunction, oxidative stress, and peptide disintegration impairments20. Nevertheless, genetic or environmental factors: both target the gap-junctions and ion-channels of the CNS to produce PD pathogenesis which has thus been reviewed in detail in this article.
Many advanced drugs namely, carbenoxolone, octanol, tonaberasat, and technologies like electroencephalogram, and electrocardiogram are being used to detect and decrease the progression of PD, albeit with incomplete cure or understating of the disease. Thus, we have discussed such drugs and their mechanisms of action in detail in this review to identify and apply newer strategies to treat PD. Overall, an emphasis on the roles of gap-junction and ion-channels in PD is given in this review thereby treating them as useful drug targets to combat this neural disorder. Therefore, the communication between neural gap-junctions and ion-channels, leading to the spread of PD, is discussed in detail and its importance for better physiological and clinical analyses are also explained.
Parkinson’s disease overview and Braak staging of Lewy pathology
PD, often known as Parkinson’s syndrome, is a protracted neurodegenerative illness that involves damage to the nervous system resulting in motor function impairment. The very first comprehensive explanation of Parkinsonism was presented almost two hundred years ago yet the concept about the progression of the disease keeps changing. Increasing depletion of a dopaminergic neuron inside the SNpc is a critical pathogenic characteristic of PD. Medical correlation analysis revealed that intermediate to extensive dopaminergic neuronal depletion within the SNpc region seems to be the starting point of motor characteristics, particularly bradykinesia and stiffness, in advanced PD7. α-synuclein protein remains in a misfolded form in PD. These proteins turn out to be hydrophobic and form subcellular clumps within the cellular body known as Lewy bodies (LBs) and further develop as Lewy neurites of neurons21. It has been postulated that Lewy pathology proceeds stereotypically within PD.
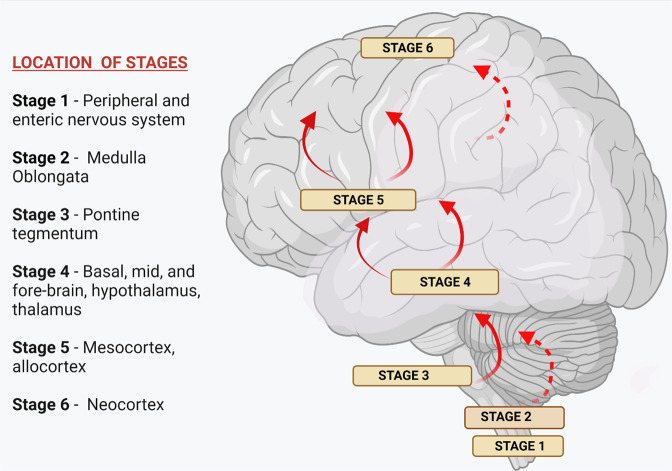
Braak and his coworkers have suggested that there are six stages of the progression of PD and it is known as Braak staging of Lewy pathology in PD or Braak hypothesis (Fig. 1). It follows the route from the peripheral nervous system (PNS) to the CNS3,22. Phases 1 and 2 might correlate to the start of premotor indications, while phase 3 indicates the presence of motor symptoms owing to nigrostriatal dopamine insufficiency, and phases 4–6 to the presence of non-motor symptoms of severe illness7 (Fig. 1).
Fig. 1. Braak-staging of Lewy pathology in PD.
Braak et al., 2003 suggested a six staged course of Parkinson’s illness203. As per this concept, α-synuclein accumulates particularly in cerebral areas as well as in neuronal areas, resulting in Lewy pathogenesis throughout a stereo typical, sequential behavior which steps up caudo-rostrally. In its first stage, Lewy pathogenesis starts through the lower midbrain (along with the dorsal motor nucleus of the vagus nerve in the medulla), then to the coeruleus-subcoeruleus complex, raphe nuclei, giganto cellular reticular nucleus in the anteromedial temporal mesocortex, cingulate cortex, and later to the neocortical structures. The illness is thought to initiate in the peripheral and spread to the CNS. With the extent of this Braak stage, the extent of infections in the sensitive areas worsens, and the illness progresses203.
Olfaction abnormalities, cognition impairments, neuropsychiatric disorders, disturbed sleep, autonomic dysfunction, ache, and exhaustion are examples of non-motor symptoms. Such indications are typical within the initial stages of PD and are linked to low standards of living23. The premotor or prodromal stage of the illness is characterized by poor olfactory, bowel problems, depressive symptoms, extreme drowsiness during the day, and quick ocular mobility sleeping behavioral disorders7. PD patients have numerous motor impairments, such as gait disruption, poor writing griping strength, even difficulty with speaking, and also a combination of cardinal abnormalities, also known as a classical triad, is formed by tremor, bradykinesia/akinesia, and rigidity/postural instability24.
Etiology of PD: environmental and genetic factors
A majority of PD cases are thought to have a complex etiology resulting from the interaction of environment and genetic variables. Pesticide contact, agricultural labor, or countryside housing have now been linked to an elevated risk of PD which has been proved in various research analyses across the globe. Agrochemicals linked to Parkinsonism, such as paraquat, rotenone, 2,4-D, and many dithiocarbamates and organochlorines, produce exploratory PD in investigational research lending support to the concept that all these correlations represent causative effects25. Employment as welders had indeed been related to an elevated danger of PD, presumably as the effect of manganese within welding gases26. Nevertheless, research on this link between welders and PD is conflicting. Additional metal ions, including irons and leads, have been linked to an increased probability of PD in exploratory in vitro and in vivo investigations25,26. Head trauma could result in a breach of blood-brain barriers, long-term head inflammatory processes, disturbance of mitochondrial function, and an upsurge in glutamine release and α-synuclein buildup within the cortex, all of which would lead to an elevated probability of Parkinsonism25. Interestingly, many of our food habits and lifestyle also induces a level of PD. People with high consumption of dairy products, alcohol, and smoking have been proved to show a linkage in the elevated level of PD during many research analyses. There are several potential hazard variables for Parkinson’s in which data is either lacking or conflicting. Initial stage determinants include the time of conception, body mass index during birth, maternal age, and numerous diseases including measles, CNS infections, hepatitis C, and Helicobacter pylori are among them. Influenza has already been linked to a higher possibility of PD27. However, mechanisms of action of these environmental factors including toxic chemicals on PD are mostly unknown so far.
Many genetic factors and their mutations have been associated with the onset of PD. The earliest gene to be identified and discovered was the SNCA gene which encodes the protein α-synuclein28. The leading mutation which is involved in the progression of PD is a missense mutation, which leads to amino acid substitution and multiplication29. This amino acid substitution helps in α-synuclein accumulation29.
Pathophysiology of Parkinson’s disease
Irrespective of the underlying pathologies (ecological, genomic, or some additional risk variables) of PD, numerous important biochemical processes and characteristics have been identified: (1) in vivo: in adult postmortem samples, human cerebral tissue and organs, and in animals models; and (2) ex vivo: in adult cell lines and cultures. Based on such findings, the pathophysiology of PD may arise from any one or combination of five main possible pathways, namely: (1) α-synuclein protein aggregation mediated pathway, (2) neuroinflammation mediated pathway, (3) neuronal abnormalities, (4) mitochondrial abnormality and mitophagy, and (5) gut-brain microbiome interaction (Figs. 2–4). Each of these pathways has specific substages as well, as discussed briefly in this section. However, the role of gap-junctions and ion-channels in PD pathophysiology is less understood or less explained elsewhere which forms the rationale for writing the current article.
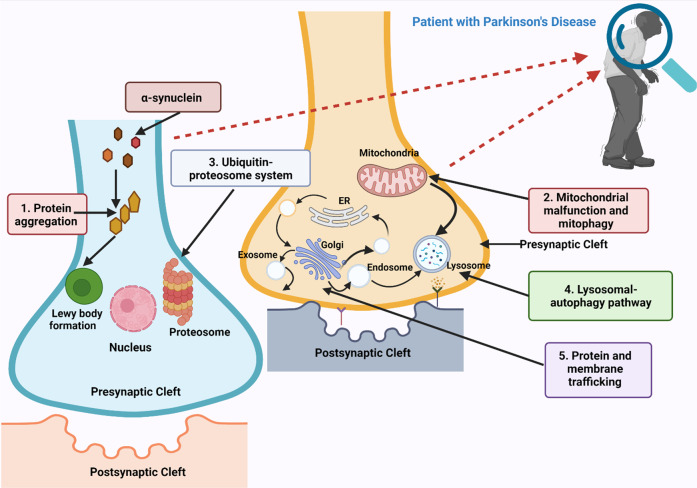
Fig. 2. Different molecular pathways that are involved in the pathogenesis of PD.
Several factors are implicated in the preferential degradation of substantia nigra neurons in the brain during Parkinsonism, involving cytotoxic peptide buildup and aggregation (1), mitochondrial malfunction (2), and oxidative stress204. The buildup of α-synuclein is an important phase in pathogenesis which is a component of the Lewy Body. Defects in the Ubiquitin-Proteasome System (3), and Lysosomal autophagy pathway (4), which usually operates as a form of protein degradation system, cause changes in protein regulation, which may encourage the extremely toxic buildup of peptides that are harmful to neurotransmission205. Neural cell apoptosis in Parkinsonism is also caused by mitochondrial malfunction, which causes an increase in oxidative stress and a decrease in ATP synthesis. Neuronal malfunction and the buildup of misfolded α-synuclein originate from impairments within protein and membrane trafficking mechanism (5)206. Similarly, alterations in intracellular sorting and disintegration have a significant impact on the neuronal trafficking of protein aggregates, enabling the cell-to-cell dissemination of dangerous α-synuclein molecules leading to increased pathological alternations in this disease. ER: Endoplasmic Reticulum.
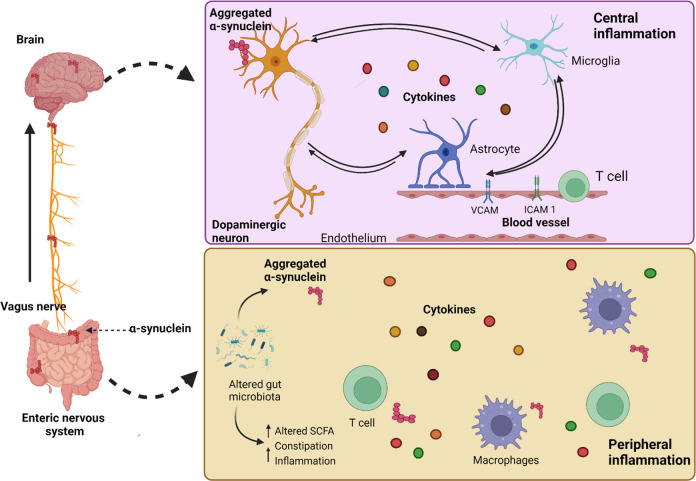
Fig. 4. Gut-brain microbiome interaction207.
Changes inside the intestinal microbiome might encourage α-synuclein accumulation as well as cause an inflammatory reaction inside the periphery. This may include elevated cytokine production and activation of T cells, according to the “gut-brain axis” concept in PD. Aggregation of α-synuclein is thought to propagate in a prion-like way initially from the peripheral to the central nervous system via the vagus nerve. When it enters the brain, proteinopathy, in combination with many invoking variables like mitochondrial impairment, ROS, etc., would then endure core inflammatory response inside a vicious spiral among dropping dead dopamine neuronal cells, glia, and activated endothelium. This will in turn get exacerbated by intruding peripheral immune cells. SCFA: Short-chain fatty acids, VCAM: Vascular Cell Adhesion Molecule, ICAM1: Intercellular Adhesion Molecule 1.
Mechanism 1: α-synuclein protein aggregation mediated pathway
The pathogenesis of PD involves various steps like an agglomeration of proteins, intercellular proteins as well as membrane translocation, ubiquitination, proteasomal pathway, and autophagosome system (Fig. 2)7. A significant discovery in recent times has shown that PD pathogenesis is mostly caused by α-synuclein via the formation of LBs as explained above. α-synuclein is currently believed to generate a multitude of distinct aggregation forms, comprising of tiny dot-like or narrow ribbon-like structures, aqueous oligomers consisting of 2–100 α-synuclein monomers30. All these alternative forms of α-synuclein protein monomers turn out to be toxic to the neurons and result in neurodegeneration in PD. In some PD patients, an inclusion body composed of different proteins is also observed frequently. β-amyloid plaques and tau-containing neurofibrillary tangles which are mainly found in patients with AD are also found in a comparable amount of PD patients.
Due to biological interconnections, α-synuclein is naturally expanded and takes on a tertiary configuration. The protein’s aberrant agglomeration has been known to be harmful to dopaminergic neurons, resulting in neurodegeneration related to PD. α-synuclein structural alterations and accumulation can be influenced by oxidative damage, PD gene mutations, and upregulation31. In PD, α-synuclein deposition in astrocytes leads to microglia owing to the production of pro-inflammatory cytokines such as tumor necrosis factor-γ (TNF-γ), interleukin-1 (IL-1), and interferon-gamma (IFN-γ)30. In that regard, microglia stimulation has also been hypothesized to be helpful for the initial stages of neurodegeneration, when microglia cells tend to remove α-synuclein of the external area resulting in neuronal discharges or induced apoptosis leading to neurodegeneration32. Long-term stimulation of microglial, on the other hand, dramatically raises the quantities of pro-inflammatory cytokine and Reactive Oxygen Species (ROS), resulting in a worsening neuronal death scenario32. It is also known that α-synuclein buildup causes oxidative damage via two adjacent trajectories: actively promoting the formation of excess ROS or indirectly interacting with the scavenging of defective mitochondria from which the bulk of ROS are formed33.
Normal humans have systems in place that prohibit proteins from misfolding and accumulating inappropriately. These proteins, for example, are collected by the lysosomal and ubiquitin-proteasome systems, and specific chaperones could rectify such misfolding aggregates34.
Ubiquitin-Proteasome System
Among eukaryotes, ubiquitin is a 76-amino-acid constituent that is bonded covalently to target specific peptide complexes for disintegration through the ubiquitin-proteasome system (UPS). Approximately three decades ago, the existence of ubiquitin-immunopositive LBs inside the cerebellum of patients with PD was discovered35. Proteasome dysfunction was eventually discovered inside the ventral striatum of PD patients36. Notably, biological research demonstrates that UPS disorder plays a role in PD pathology. Similarly, PD-associated gene variability on PARK237 and UCHL, which encapsulate the ubiquitin E3 ligase parkin and the deubiquitinating enzyme UCHL1, respectively, have also been identified37. Among healthy tissues, the UPS represents highly effective cellular clearance machinery, even though it is primarily accountable for the breakdown of shorter polypeptides into tiny cytoplasmic and plasma membrane peptides36. In the cytosol, nuclei, and endoplasm, it is deemed essential in the destruction of unfolded peptides or defective peptides38. In the etiology of PD, disruption or breakdown of this essential biological machinery has resulted in the accumulation of unfolded amyloid proteins like LB as well as an upsurge in neurotoxicity within SNpc38. Numerous additional proteins, including parkin and UCH-L1, as well as UPS, are implicated in the breakdown of misfolded α-synuclein during PD38. UCH-L1 is implicated in the synthesis of ubiquitin, which is co-localized with LB38.
Lysosomal autophagy pathway
Autophagy, which comprises macro-, micro-, and chaperone-mediated autophagy (CMA), are specialized systems that act as alternate peptide clearing machines within each cell, allowing LBs to be degraded in PD38,39. Particularly under relaxing circumstances, micro-autophagy is primarily involved in the destruction of tiny cytoplasmic proteins, whereas macro-autophagy is accountable for the breakdown of larger complexes. CMA is more specific since it interacts with heat-shock cognate protein (HSC70), which binds to tiny soluble proteins and degrades them via a unique pentapeptide targeting motif (KFERQ)38. Several lysosome and autophagy-related elements are dysfunctional or differently regulated in PD, analogous to results with the UPS mechanism. The autophagosome marker LC3-II was found to be elevated in substantia nigra neurons of the PD brain, indicating aggregation of autophagic vacuoles40. In other assessments, however, key lysosomal proteins (LAMP1 and LAMP2A) and various heat shock protein family molecular chaperones (such as HSC70 and HSP35) were discovered to be diminished40. The α-synuclein has just been demonstrated to be preferentially translocated towards lysosomes, where it is degraded by the CMA38. As a result, CMA malfunction reduces the effectiveness of α-synuclein degradation, resulting in an overabundance of this protein and a considerable reduction in neurotransmission38.
Protein and membrane trafficking mechanism
Cell membrane trafficking is also a type of intracellular transportation wherein larger components within transportation vesicles seem to be capable of reaching their intended locations despite trying to cross membranes38,41. As a result, membrane trafficking is important not only for regulating cell equilibrium but also for meeting unique needs throughout growth, differentiating, signaling interpretation, and transmission. Improper endocytosis trafficking, comprising mutations or aberrant production of key endocytosis elements, are also significantly linked to PD42. This includes the retromer subunit VPS35 and the retromer-associated protein receptor-mediated endocytosis 8 (RME-8)43, auxilin41, and synaptojanin41. Endocytosis trafficking, like AD, can cause PD disease in a variety of ways, including impacting pathogenic α-synuclein aggregate intake, modifying synaptic vesicles or neurotransmitter receptors traffic, and disrupting lysosome equilibrium and autophagy41. Disorders in various trafficking pathways may cause PD pathogenesis. LRRK2, which is disrupted in 1% of sporadic and 5% of familial PD cases, is a finding of recent studies41. LRRK2 phosphorylates numerous RAB GTPases across multiple trafficked stages44, while more prevalent PD mutations activate LRRK241. Excess LRRK2-mediated activation impairs these RABs’ capacity to interact with transcription elements and functional molecules, causing traffic disruption44. RAB29 increases LRRK2 activity at cellular membranes and is associated with PD independently, implying that such protein (co)operates in a similar disease mechanism41.
Mechanism 2: neuroinflammation
Another mode of PD pathogenesis is neuroinflammation (Fig. 3). The presence of a severe inflammatory response within the brain is mostly regulated by localized astrocytes. Although the presence of astrocytes and microglia have been increasingly identified in PD, it has remained a less explored area to date. Microglia and astrocytes generally seem to be engaged in the clearing of external material, that may help neurons endure. Active microglia can produce neurotrophic substances like brain-derived neurotrophic factor and glial-derived neurotrophic factor, but they might also produce damaging reactive oxygen and nitrogen species (ROS and NOS) and pro-inflammatory cytokines thereby leading to PD7. In PD patients, various innate and adaptive immune response aberrations have been identified, along with an upsurge in proinflammatory cytokines and a modified intracellular community (such as monocytes and their precursors)31. Clinically randomized trials indicate a connection between autoimmune illnesses and PD. An indication of inflammatory cellular activity (such as microglia) on molecular neuroimaging and neuroinflammation characteristics in experimental PD models has shown a confirmation of this mechanism.
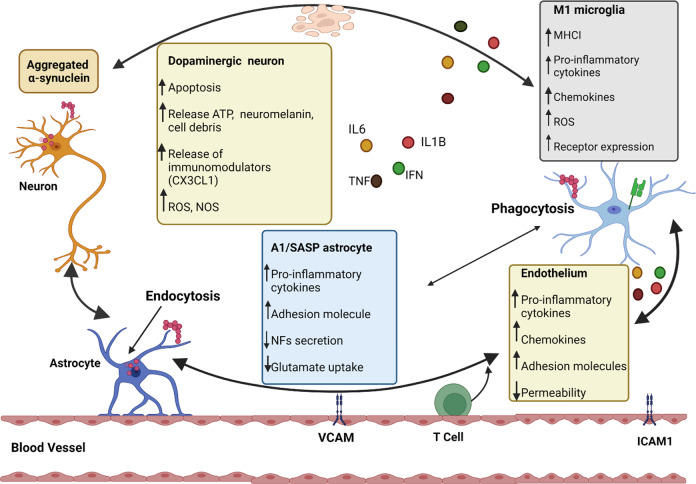
Fig. 3. Mechanism of neuroinflammation207 in PD.
Interaction among various cell groups inside the cortex causes inflammation is depicted here. Neurons, astrocytes, microglia, and endothelial cells are vulnerable to α-synuclein aggregation (i.e., through phagocytic cells, endocytic pathway, Toll-like receptor (TLR) activation, etc.), which could indeed hinder their homeostatic operations (diminished secretion of neurotrophic factors (NFs), deficient glutamate uptake, etc.) as well as production of proinflammatory cytokines and chemokines (MHCI in microglial cells, adhesion molecules in the endothelium, etc.). Peripheral immune cells (such as CD4+ T cells) are also drawn into the cerebral tissue. The presence of these immunoregulatory factors, as well as the lack of effective relieving processes, adds to the inflammation milieu. VCAM: Vascular Cell Adhesion Protein, ICAM1: Intercellular Adhesion Molecule 1, NOS: Nitric oxide species, ROS: Reactive Oxygen Species, SASP: Senescence-associated secretory phenotype.
Mechanism 3: neuronal abnormality
Another alternate pathway of PD pathogenesis involves loss of dopaminergic neurons in the substantial nigra-striatum in the patients’ brain16. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-model of PD exhibited an increase in expression of specific connexins (discussed in detail in two separate sections below) in the striatum, although the coupling of astrocytes was not increased13. Change or malfunction of gap-junctions in the brains of PD patients have not been reported yet thereby its pathological role in PD is ambiguous so far. Therefore, the mechanism and significance of gap-junctions in PD pathogenesis have been explored in detail in this article. Although most of the mechanisms of tremors and dyskinesis which are commonly observed in PD patients are still obscure, the inferior olive has been focused on as the pathological generator of tremors14,15. Neurons of the inferior olive are electrically coupled through gap-junctions which play a role in creating oscillatory activity. Some studies have reported that the GJIC of inferior olive neurons is responsible for tremors13,14. All of these play a major role in the spreading of PD. These significant hallmarks are frequently correlated with several other interconnected events, such as vesicles transit interruption, the decline of tubulin integrity, nerve cell inflammation, trophic factor disruption, iron metabolic pathway dysregulation, endoplasmic reticulum impairment, poly (ADP-ribose) polymerase, and other enzymatic activation, to name a few31.
Mechanism 4: Mitochondrial malfunction and mitophagy
Mitochondria are the “powerhouses” of cells, producing adenosine triphosphate (ATP) via oxidative phosphorylation45. ROS are produced by the electron transport chain during the ATP generation process. Radicals from complex I are transferred to the mitochondrial matrix, but radicals from complex III are delivered to both the inner membrane space (IMS) and the mitochondrial matrix33. Reduced mitochondrial complex I activity is also reported in PD patients, additionally, the utilization of its inhibitor (e.g., rotenone) is believed to trigger mitochondrial destruction (such as reduced mitochondrial prospects, cytochrome C release, initiation of the caspases, and eventual induction of apoptosis) in exploratory PD models31. Mitochondrial malfunction increases oxidative stress and ROS generation; which in turn, are damaging to the electron transport system, contributing to more ROS production33. It was proposed that mitochondria-induced ROS overconsumption plays a significant role in apoptosis and the development of delayed neurological diseases, notably in idiopathic PD46. Throughout the cerebral regions of PD patients, there is significant lipid peroxidation, glutathione deprivation, and an elevation in protein oxidation34. Dopaminergic oxidation results in the production of serotonin quinone, which can significantly affect proteins. Mitochondrial failure causes a lack of ATP, which is required for dopaminergic neurons to transmit electric impulses, retain ionic gradients, and release dopamine33. All these can lead to PD pathogenesis.
Mechanism 5: gut-brain microbiome interaction
There is an increasing amount of information that reveals the gut-brain connection plays a role in PD etiology, with the vagus nerves acting as an “expressway” for accumulated α-synuclein to go from the intestinal system to the lower brainstem (Fig. 4)47. Some reports show that cells obtained from PD patients that were implanted into recombinant α-synuclein expressing rats caused motor impairments, and antibiotics therapy restored some of the problems, whereas microbial recolonization exacerbated the pathogenesis48. Moreover, numerous investigations have indicated that vagotomy and appendectomy may lower the chance of acquiring PD. However, more research is required to fully understand the involvement of the gut tract and microbiomes, infections, and inflammatory responses in causing α-synuclein aggregate and spreading to the nervous system as a pathological process for PD.
Why is it important to study gap-junctions and ion-channels in the context of PD?
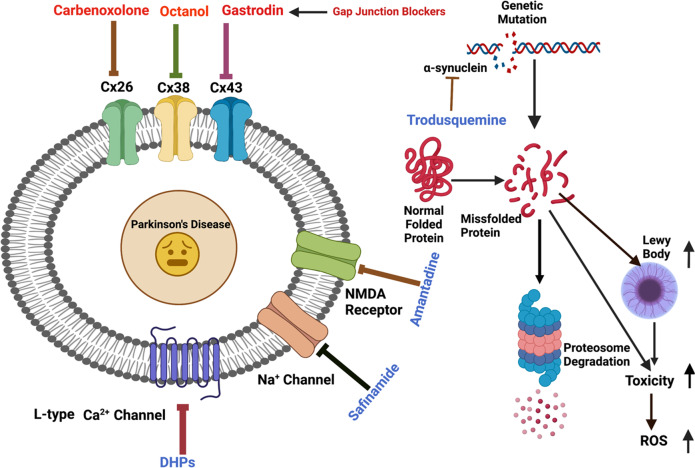
Gap-junction (GJ) channels and associated connexins (Cxs) are complicated proteins that play critical roles in the nervous system and neural cellular interaction medium. Neurons, astrocytes, oligodendrocytes, and microglial cells express a diverse set of Cxs which are required during intercellular interaction and the transport of small molecules, ions, as well as neuronal and glial transmitters17. They not only play an important function in the CNS but also play a pathological role in many neurodegenerative diseases like PD, AD, and HD49. However, their exact role and mechanism of action in the case of PD are scarcely explored so far, which otherwise is very important to not only understand the disease pathology but also to develop a treatment for it. Since PD is associated with the development of astrocytosis and gliosis, the elevation in connexin synthesis in astrocytes and microglial cells is expected49. The most common connexin variation in inflammation circumstances, i.e. Cx43, is reported to be elevated in PD. The production of inflammation chemicals by activated microglia disrupts capillaries and produces extremely unstable vessels. Furthermore, active microglia enhance connexin hemichannel activation, culminating in a bilateral flow among the external and internal cellular compartments50. Thus, it is clear that different Cxs play different roles in connection to PD as discussed in detail in a separate section below which forms the origin of writing the current review article to explore gap-junctions in terms of their mechanism of action and plausible use of them as targets for therapeutic interventions in PD.
Similarly, multiple ion-channels work together to control the secretion of dopaminergic as well as the firing action of substantia nigra neurons. The abnormal transport of different ions within the internal environment is caused by the deregulation of these systems. This disrupts intracellular signaling pathways, cellular equilibrium, and energy metabolism. This indicates that ion-channels also play a critical role in the elevated sensitivity of dopaminergic neurons to degeneration in PD. Blocking ion-channels is also an appealing mechanistic way to combat the progression of neurotoxicity and thus has been explained in detail in yet another section below.
Different types of gap-junctions: their mechanism of action and functions in relation to PD
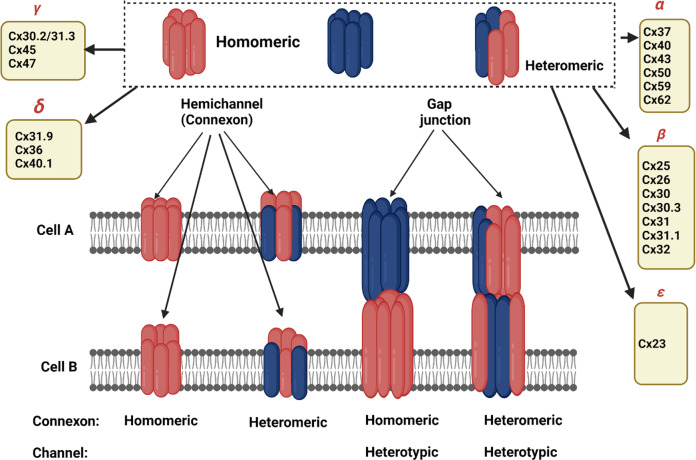
Gap-junctions (GJs) have complicated arrangements made up of homomeric or heteromeric hexamers of connexin (Cx), connexons (hemichannels (HCs)) that are situated side by side of plasma membranes of two neighboring cells51. Connexins create hemichannels, or connexons, within clusters of six, and two hemichannels join to produce gap-junctions. Each solitary GJ channel consists of two opposed hemichannels termed connexons, and are typically comprised of six proteins called connexins52 as depicted in Fig. 5. Different cell types in the mammalian brain express at least ten different types of connexion which in turn makes the organism to be diversified and forms complex intercellular communications. Connexins, one on either side, could be classified under subclasses α, β, γ, δ, ε, depending on the quantity of homology and the extent of the cytoplasmic loop as mentioned in Fig. 5 and Table 1. Astrocyte expresses: Cx43, Cx30, Cx26; oligodendrocyte expresses: Cx32, Cx29, Cx47; microglia express: Cx43, Cx32, Cx36; and all endocytic cells express three connexins: Cx37, Cx40, and Cx4352.
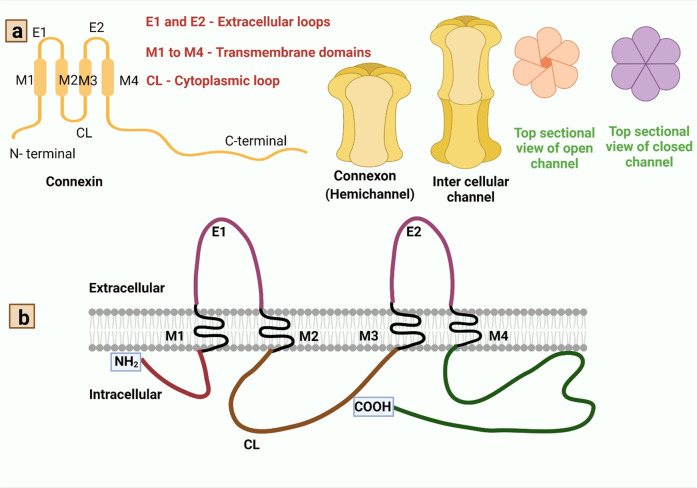
Fig. 5. Structural organization of connexins and connexons to form gap-junction.
Panel a: Gap-junctions are made up of homomeric or heteromeric hexamers of connexin, connexon (hemichannels) that are situated side by side in the plasma membranes of two neighboring cells51. Connexins have four strongly organized transmembrane segments (TMSs), with predominantly unstructured C and N cytosolic termini, a cytoplasmic loop (CL), and two extracellular loops (EL-1) and (EL-2). Connexins create hemichannels, or connexons, within clusters of six, and two hemichannels join to produce gap-junctions. Panel b: The connexin polypeptide crosses across the membranes four times (black), exposing its amino-terminal and carboxy-terminal regions to the cytosol and connecting via two extracellular and one intracellularly exposed loops208. The extremely consistent amino-terminal domain (maroon) regulates its channeling pore whereas the two invariant disulfide-linked external loops (magenta) regulate hemichannel coupling among neighboring channels209. The length of the cytoplasmic loop (brown) differs widely amongst connexin subclasses210. Within the connexin familial member, the span of the cytoplasm terminal (deep green) and its amino acid composition are highly variable211.
Table 1.
| Cellular/Gap-Junctions | Connexin | Subgroups |
| Astrocytes | Cx26 | β |
| Cx30 | β | |
| Cx40 | α | |
| Cx43 | α | |
| Cx45 | γ | |
| Oligodendrocytes | Cx29 | n.a. |
| Cx32 | β | |
| Cx36 | δ | |
| Cx37 | α | |
| Cx40 | α | |
| Cx43 | α | |
| Cx45 | γ | |
| Cx47 | γ | |
| Neuronal Precursors | Cx26 | β |
| Cx33 | α | |
| Cx40 | α | |
| Cx43 | α | |
| Cx30.3 | β | |
| Cx31 | β | |
| Cx46 | α | |
| Cx50 | α | |
| Cx57 | α |
*n.a.= not available.
Cx37 and Cx45, like Cx43, are substantially upregulated in PD, but Cx26 and Cx25 are negatively regulated49. In PD, increased production of Cx37, Cx43, and Cx45 in cerebral endothelial cells has been linked to capillary leaking and improved endothelial cell interaction49. Cx47 and Cx32 are dysregulated in PD, although Cx30.2 stays unaltered. In neurological illness, these three oligodendrocyte connexins were associated with neuronal damage49. Cx31.9, a protein generated in the cerebrum, was also discovered to be deregulated in PD patients49.
Homotypic Gap-Junctions
Between neurons
So far, eight distinct Cxs were identified in specific neuron cell lines and examined ex vivo and in vivo in developing animal models. As seen in Table 1, the majority of these Cxs are present in a wide range of cells and therefore are not confined only to neuronal cells only. Several investigations have shown that neurons express Cx32 and Cx43 (Table 1). Cx36 expression is constrained to neuronal, according to the latest survey whereas Cx32 and Cx43 had been identified in oligodendrocytes and astrocytes, correspondingly53. Cx36 is the first Cx variant to be generated solely in CNS nerve cells and it has unusual functioning features in addition to other Cxs54.
Between astrocytes
Astrocytes have a syncytium arrangement because of their extensive abundance with tight junctions which allows for rapid interstitial transport of molecules and transmitting elements. Intracellular interactions within cultivated astrocytes are regulated via tight junctions constituted predominantly of Cx43. Consequently, modest quantities of Cx30, Cx40, and Cx45 had been observed55–57. Cx43 production, as well as linkage efficacy, differs among culture astrocytes, generated from various cerebral regions, for example, being modest among mouse striatum and high in the hypothalamus region of astrocytes58. Such variations might point to a diverse localization of Cx43 in the cerebrum. Furthermore, evidence suggests that the expression of Cx30 is more in the adult gray matter of astrocytes, while the respective amounts of Cx43 and Cx30 fluctuate depending upon the stage of development as well as the site examined59. Cx26 previously assumed to exist only aberrantly inside cerebral parenchyma has lately been detected within cellular/gap-junctions of astrocytes60.
Oligodendrocytes
In vivo and ex vivo studies suggest that there are expressions of connexin genes Cx32 and Cx45 in oligodendrocytes. Cx29, a recently identified connexin, has been found in oligodendrocytes. Various immunofluorescent studies indicate that newly generated connexin Cx45 was expressed in oligodendrocytes61–63.
Microglial, ependymal, and meningeal cells
Lately, the expression of connexins within microglia cell lines was revealed, and modest amounts of Cx43 were reported. Cx43 expression gets elevated after microglia cells get activated. Cx43 is located just at boundaries among activated glial cells and also they get coupled with dye with gap-junctions in the subsequent situation64. Cx26 and Cx43 had also been reported in epithelial cells (ependymal cells) of ventricles in cerebrum65,66. Gap-junctions are prevalent across the growing and mature meninges. In meningeal cells, the three connexins which have elevated expressions are Cx26, Cx30, and Cx4360,67.
Heterotypic Gap-Junctions
Within microglial cell lines
Although primary Cx reported is Cx43 in astrocytes and Cx32 in oligodendrocytes, which are probable donors to both sides of its channels. Conversely, Cx43 and Cx45 could indeed communicate together and may generate heterogeneous linkages68.
Between glia and neurons
Here, the identity of connexin implicated throughout this heterotypic junction is unknown. Expression of connexin in glial cells are Cx26, Cx32, and Cx43, and the expressions of connexins in neuron are Cx26 and Cx32. An investigation of more than 5000 connections comprised of various Cxs (i.e., Cx30, Cx32, Cx36, and Cx43) from numerous cerebral areas revealed tight channels between astrocytes and neurons69.
Connexin Hemichannel
Under standard conditions, Cx hemichannels need not be active. It stops particles and relatively small compounds from entering the cell and also cytoplasm ingredient spillage, which might lead to cytoplasm deficiency of important molecules. Cx43 hemichannels may activate within cerebral astrocytes following membrane disruption exposition towards a reduced calcium ionic medium, or metabolism limitation. Immune cells may be able to save deceased ones through exchanging molecules as well as critical compounds across complete Cx43 cell signaling junctions.
What is known about the role of gap-junctions in the spread of PD pathology?
Although all Cxs are crucial for intercellular signaling inside the brains, recent research has revealed that Cx30, Cx36, and Cx43 play key functions in cellular regulation, neurodegenerative processes, and neuroprotective properties. Cx30, one of the major Cxs found in astrocytes, plays an important function during astrocyte-astrocyte communications and nutrition transportation70. Moreover, α-synuclein has been shown to increase the production of Cx43 HCs (hemichannels) in cortical astrocytes, resulting in an increased flow of calcium ions in addition to other effects like activating cytokines, cyclooxygenase 2, and inducible nitric oxide synthase as shown in Fig. 671. Rodent models of PD were experimentally utilized to determine if Cx-mediated HCs perform a critical function for the astrocyte malfunction. The most common neurotoxin chemicals which cause degeneration mechanistically modulate Cx43 HCs include 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), and rotenone72. MPTP produces a rapid, although temporary rise in the striatum Cx43 mRNA, which is accompanied by a persistent elevation in Cx43 immunoreactive puncta73. Likewise, using an in vitro and in vivo rotenone-induced model of PD, higher levels of Cx43 were found alongside elevated GJIC, which was also again confirmed within a mouse PD model with an observed rise in phosphorylated Cx43 which is required for GJIC72. The elevated level of phosphorylated Cx43 was observed in Striatum and Hippocampus regions. All these studies have shown an increase in GJIC with an increment in Cx43 level in astrocytes72. Such a surge in Cx43 overexpression resulted in higher HC functioning, improved GJ interaction, plus heightened cytoplasmic Ca2+ concentrations, all of which contribute to neurotoxicity and neurodegeneration30. Other experiments employing rotenone demonstrated a downregulation of Cx43 resulting in decreased GJ porosity in primary culture astrocytes, indicating that astrocytic GJ malfunctioning might be involved in underlying PD pathogenesis74. Moreover, studies involving 6-OHDA-induced mouse models of PD resulted in significant levels of Cx30, but hardly Cx43, throughout the stria; however, levels of Cx43 and Cx30 have significantly increased surrounding arteries, indicating enhanced metabolic interaction75. Cx30 and Cx43 were found to be increased inside the stria of an acute PD animal model induced by MPTP treatment. Furthermore, MPTP therapy has been demonstrated in a Cx30 KO animal model to increase the overall depletion of dopaminergic neurons and downregulate the S100a10 genes, which is crucial for cell motility, cell to cell translocation. This in turn represses GFAP and astrogliosis within the striatum76. These findings imply that Cx30 insufficiency reduces the neuroprotective role of astrocytes within stria and thus enhancing Cx30 functions which is recommended as a treatment option for PD patients30. Cx36 GJ channels vary from other Cx variants in terms of regulation characteristics, like reduced unified conductivity as well as susceptibility to transjunctional voltages; alterations related with any of these has a regulatory roles in PD77. GJs were subsequently suggested to be implicated in the internal process of synchronizing, owing to a potential GJ reconfiguration and GJ connectivity linked to dopamine levels, wherein GJ conductivity decreases as dopamine levels increases as shown in Fig. 7.
Fig. 6. Pictorial depiction showing that connexins can be arranged in many different ways in a gap-junction channel.
The diagram depicts several constituents of the gap-junction channel. A particular kind of connexin forms homomeric connexons. Heteromeric connexons are made up of greater than a single connexin kind. Whenever connexons of a similar structure create a gap-junction channel, it is referred to be a homotypic channel. Whenever the constituents of the connexons vary, the channels are known as heterotypic. Depending on homology, the connexin group is classified into five subgroups (α, β, γ, δ, and ε)211. Connexins oligomerize into homomeric or heteromeric hexamers alongside adjacent connexins of a similar subtype as well as on rare occasions, with connexins of different subtypes, resulting in a very diverse channel configurations211.
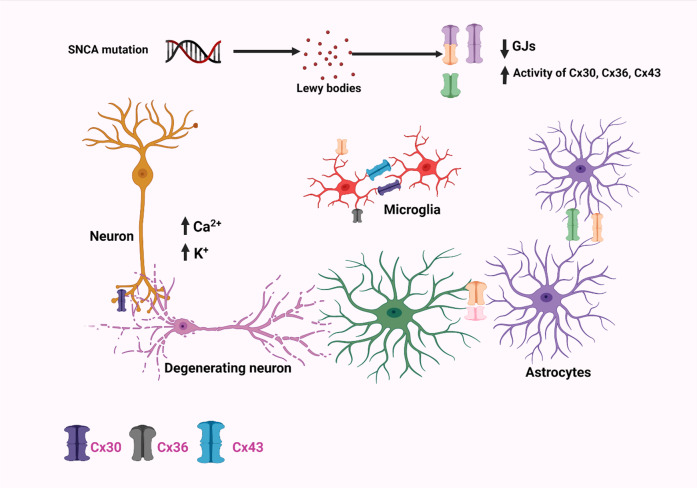
Fig. 7. Pictorial depiction of major changes occurring in the regulation of Cx30, Cx36, and Cx43 in neurons, astrocytes, and microglia, respectively in a PD patient.
SNCA mutation leads to the accumulation of Lewy bodies which in turn increases the opening of Cx43 HCs, thereby resulting in elevated internalized Ca2+ levels and cytokine production. SNCA: Synuclein Alpha gene.
Altogether, these findings demonstrate the potential impacts of Cx30, Cx36, and Cx43 on PD and in the foreseeable future, it might be useful to focus on these gap-junction regulations in addition to chemical therapies for improving neurocognitive impairments in PD patients.
Different types of ion-channels, their mechanism of action, and their functions concerning PD
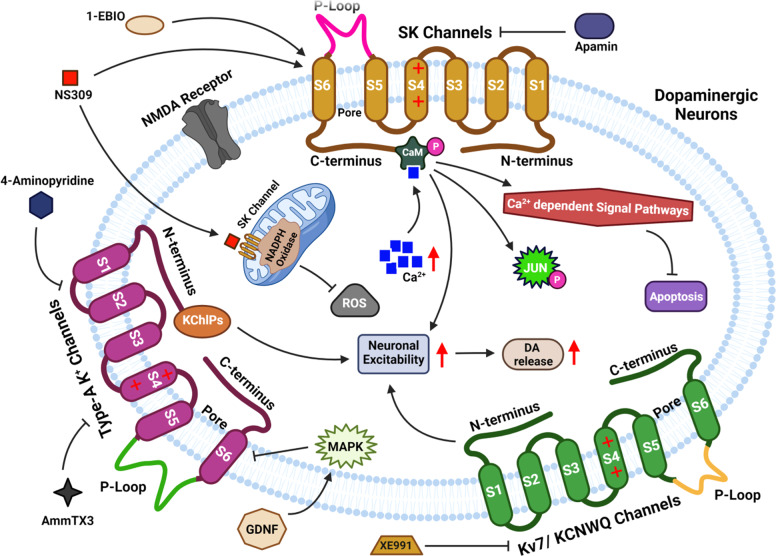
Dopaminergic Neurons (DA) form the bilateral dopaminergic pathway which is identified by their ability to transfer current from irregular tone or the activity played by the pacemaker to the bursting out of the activity within or outside an organism78. Ion-channels control their firing action potential and the pivot-like activity of dopamine, released from the axonal terminus of subcortical basal ganglia, functions like a motor18,79. Positively charged potassium is responsible for maintaining a balance and equilibrium within the cell thereby maintaining its volume and at the same time maintaining the potential of the cellular membrane18,79 (Fig. 8). Depending on the sequences of amino acids, K+ channels can be classified into three major classes: (1) voltage-gated K+ (Kv) channels, (2) Kir channels which rectify the inward activities such as Kir1 − 7 and KATP channels, (3) K2P channels with two pores such as K2P15–K2P18, K2P9–K2P10, KCNK, K2P12–K2P13, and K2P1–K2P7. For their vast roles in neuronal activities, potassium ions are considered one of the important ions in PD.
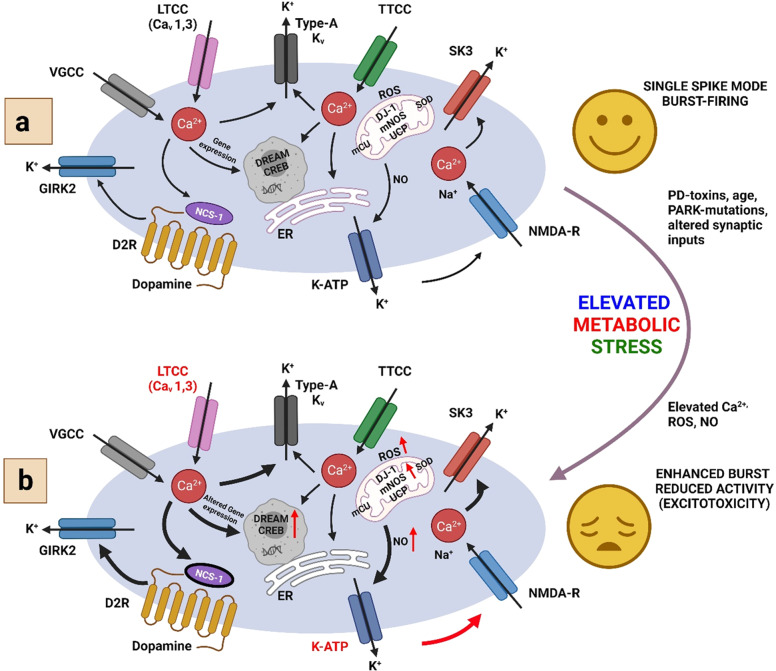
Fig. 8. Pictorial depiction of different types of ion-channels in the CNS and their mechanism of action under unstressed (Panel a) and stressed (Panel b) conditions.
Panel a: Mechanistic view of networking of ion-channels in SN dopamine nerve cells in PD and other health states. Under physiological conditions, activity-dependent Ca2+ loads due to (1) active participation of LTCC (L-type Calcium Chanel) and VGCC (Voltage-Gated Calcium Channel), with the help of KATP/NMDA-R, which mediates bursting activity and Ca2+-stimulated TCA (Tri Carboxylic Acid) cycle and m-NOS, (2) generated ROS for SN DA, which is controlled by ER (Endoplasmic Reticulum), mitochondrial Ca2+-buffering, UCP, ion-channels that reduces electrical activities like D2-AR/NCS1/GIRK-2, KATP, SK-3, anti-oxidative enzyme kinetics such as DJ1, SOD (Superoxide dismutase) function, and (3) Ca2+ dependent gene expression. Panel b: As the relative level of Ca2+, ROS, NO (Nitric Oxide), and metabolic stress increases, the controlled mechanism may not be enough for altered SN dopamine activity, altered gene expression to remain under physiological regulation thereby leading to apoptosis. TTCC: T-type calcium channel, DJ-1: Mutations in DJ-1 (PARK7), D2R: Dopamine Receptor, ROS: Reactive Oxygen Species, GIRK-2: G-protein-coupled inwardly rectifying potassium (GIRK) channel.
Voltage-Gated K+ (Kv) channels
These channels are responsible for the efflux of K+ and control the potential of the cell membrane via a change of voltage. Kv channel tetramers are composed of homomers and heteromers of four subunits, which comprise Hexa-transmembrane segments (S1-S6). Interaction of Kv4.3 with K-Chip3 generates type AK+ currents, which inactivates the components of the extracellular K+ currents located in SNc dopaminergic nerves. Type AK+ channels in dopaminergic nerves of substantia nigra contribute to pacemaker-controlling tonic activities80,81. Type-AK+ channel in mesencephalon dopaminergic nerve cells activates mitogen-activated kinases, thereby suggesting type-AK+ channels to be a promising diagnostic marker in Parkinsonism80,82.
Delayed rectifier K+ channels
Delayed rectifier K+ channels are a group of slow opening and closing voltage-gated K+ channels, including Kv1.1–1.6, Kv2.1–2.2, and Kv3.1–3.380,83. The increase in the expression of surface Kv2.1 channels led to the downfall of DA in the striatum terminals of DA and SN84. When the Kv2.1 channels were inserted into the cellular membranes, it led to a decrease in the concentration of K+ activating enzymes like nucleases and proteases intracellularly, leading to cell death85. With this increase, the intracellular concentration of Ca2+ increases, thus promoting the influx of Ca2+ into the cell, leading to the damage of the cell and thereby linking with the flow of K+ inside the cell, hence causing diseases associated with CNS86. The projections from the medium spiny neurons of the caudate and putamen to the internal segment of the globus pallidus and substantia nigra pars reticulata are part of a “direct pathway”. The GP is an interconnection of all major parts of the microcircuit of the basal ganglia. Abnormal discharge of high-frequency leads to stiffness and tremors in PD87. The DA nerves in PD gets degenerate during the inflammatory process of the basal ganglion. The Stichodactyla helianthus K+ channel acts as a blocking toxin for the Kv1.3 channels, which suppresses microglial cell and T-lymphocyte activation in PD88. The current generated by K+ mediates Kv1.3 channel depolarization via robust negative feedback, thus proscribing SCIN sencitability89. In the striatum of PD mice, acetylcholine release from cholinergic interneurons decreases the excitability of Kv1.3 current inhibition, thereby leading to the prevention of change in acetylcholine concentration in the striatum90.
ERG K+ channels
Kv11 or ERG K+ channels belong to the family of Kv channels which encodes three different genes e.g., eag, elk, and erg genes91. This channel modulates the functioning of STN neurons. Recent developments showed that the inhibitors of ERG channels reduce STN neuron burst discharge and improper functioning in PD mice, likely E-4031 and dofetilide, whereas PD-118057 acts vice-versa92. The malfunctioning of STN neurons is associated with the change in neuropsychiatry, and antipsychotics partly block the ERG channel inside nerves, thus affecting the cells. Therefore, these channels provide unique methods for PD treatment and treatment of other neurodegenerative disorders93.
GIRK channels
GIRK channel is a part of the vast family of channels, especially Kir1–7. These types of channels mediate the synaptic action of a large number of nerves in the CNS, which shows pivotal activity after the inhibition of synapses is completed. Four genes were found in mammals that were structurally similar: GIRK1, GIRK2, GIRK3, and GIRK494. Hippocampus is that region of the brain where the GIRK2 is majorly expressed, followed by the amygdala and Substancia nigra95. The Purkinje cells and VP (ventral pallidum) neurons are some examples where the GIRK4 channels are less populated95. Auto somatodendritic D2 receptor activates the GIRK channels96.
KATP channels
KATP channels comprise of two basic units which differ structurally, like Kir6.1 − 6.2, which is the pore-forming unit of the Kir6 channel, and sulfonyl-urea receptors, which include SUR1, SUR2, SUR2A, and SUR2B. These channels are responsible for the identification of measure of energy units in the cellular components, thereby forming a combination of membrane excitation and metabolism of the cell97. The effects of oxidative stress, mitochondrial damage, and decreased ATP concentration in the DA nerves of SN, leads to the activation of the channel and hyperpolarization of the cellular membrane thus protecting the CNS by reducing excitability in the nerves. Studies have shown that the Kir6.2/SUR1 channel has been functionally expressed by SN DA neurons and the VTA. However, a selective increment was observed in SUR1 expression in the Substance nigra of MPTP mice model of PD97. It was also observed in rodents and some cases of PD that high levels of UCP, present in the inner lining of mitochondria express VTA DA neurons, and even a minute uncoupling can result in the reduction of ROS formation thereby decreasing the opening of KATP channel98. Lower concentrations of UCP-2 in SN DA neurons showed that the channels remain sensitive to metabolism and thus the possibility of KATP channels remaining open is highly increased97.
K2P channels
Identified in the name of “leakage” or “background” channels, the K2P channels depend on K+ level outside the cell. It has four transmembrane domains and two P regions99. A vital K2P channel functional in the CNS is TREK-1100, which maintains an imbalance of electrical charges between the interior of electrically excitable neurons, strongly influencing the outward movement of current and maintaining the propensity of neurons by electric charge shift101. TREK-1 was shown to have been activated by riluzole, whose characteristics are similar to NDMA receptors, attenuates L-DOPA induced unusual involuntary movement in rats, which is further reduced by CREB1 induced L-DOPA receptors102. Low-intensity pulsed ultrasound prevents or delays MPP+ induced DA neurons are degenerated by low-intensity ultrasound, K2P channel activation, and downward movement of ion-channels103.
SK channels
SK channel is a complex macromolecule that forms an important pore and gets activated with the increased level of Ca2+ in the cytoplasm. SK channel forms a four-membered family from SK1–4104. SK1–2 express themselves in the brain tissues that can alter the membrane potential, especially the cortex and hippocampus. SK3 express themselves in the tissues of secondary concern or sub-cortical areas, like the locus coeruleus, dorsal raphe, and SN, whereas SK4 channels occur in tissues of the heart, lungs, and placenta105. Constitutive binding of calmodulin to the closer proximity of C-terminus detects the action of Ca2+. Phosphorylated calmodulin influences susceptibility of SK channel. Calmodulin after binging to Ca2+, activates SK channels leading to a change in the membrane potential of a cell, thereby reducing its excitation105 (Fig. 8).
Voltage-Gated Ca2+ channels
Voltage-gated Ca2+ channel (VG-Ca2+channel) forms the main components of hyperpolarization. These members of VG-Ca2+ channels play an important role in cell signaling. CaV1 sub-member of this family of Ca2+-channels influences cells to contract, secrete, regulate expression of genes, integrate, and transmit synapses in nerve cells. The next member in this family is CaV2 which initiates neuron communication with a target cell across fast synapses. The third sub-member is CaV3 which is important for sending the electrical signal down the axon, thalamic neuron, and muscle cells of the heart106.
What is known about the role of ion-channels in the spread of PD pathology?
Roles of Kv-7/KCNQ channels
Membrane currents are the subliminal voltage-gated K+ currents originating from the Kv-7/K-CNQ channel. The K-CNQ families form a Penta-member of K-CNQ1–K-CNQ5 and Kv-7.1–Kv-7.5, whose expression is high in the CNS and PNS. K-CNQ2 and K-CNQ4 confine specific areas of SNs and VTA (Ventral Tegmental Area) of the mesencephalon107,108. Current modulates the firing frequency of dopaminergic nerves in the midbrain. K-CNQ channel activators induce hyper-polarization of dopaminergic nerve cells and inhibit synaptically induced excitation. Kv-7 channels are positioned at both presynaptic and postsynaptic sites of the striatum, counteracting the high excitatory inhibition of dopamine-D2 receptors109. XE-991 enhanced supraliminal synapses and depolarizes the projected nerves of GABA in the striatum110. XE-991was administered into the SNc, which reduced catalepsy obtained by haloperidol insertion111. XE-991 also contains protective abilities against 6-OHDA induced degradation of SN striatum. It was proven that the SK channel, Type-A K+ channel, and Kv-7/K-CNQ channel form the important substrate for PD diagnosis. Blockers/activators of the three K+ channel protects DA nerve cells in SNc, modulates neuron excitation, influences release of DA, and attenuates motor-symptoms. K-CNQ channel-opener, retigabine, downregulated L-DOPA induced dyskinesia in 6-OHDA lesioned rodents. Increased ability of GABA in the nerves of the striatum are required for the treatment of motor-fluctuation and dyskinesiasis, with the help of L-DOPA, thus reducing MSN-activity in the striatum112.
Role of GIRK channels
GIRK2 influences the generation of GIRK current in nerves, thereby decreasing the excitation of nerves. D2 receptor proteins release the G-protein β-γ, when dopamine binds, thus keeping the GIRK2 channels open113. When the GIRK2 activates, it causes a change in the membrane potential of neurons inhibiting a negative response leading to a decrease in dopamine production. However, any sort of GIRK2 mutations in rodents could be the cause of gradual deterioration of the dopaminergic nerves in the Substancia nigra, leading to abnormal stabilization and tremor developments114. L-DOPA, a type of dopaminergic pro-drug is top-rated for PD diagnosis. Studies have shown that DOPA-quinones derived from 3,4-dihydroxyphenylalanine metabolism help in the association of α-synuclein protein, abnormality in functions of mitochondria, and protein degradation115. Synthesis of L-DOPA-quinone from 2-IBA is necessary for the stabilization of GIRK channels and counteracting neuron inhibition of SN DA nerves when they bind to the Cys residues of GIRK2 channels115.
Roles of K-ATP channels
It has been observed that in MPP+ induced inhibitory action of mitochondrial respiratory chain complex-I, the SN DA neuron with the high level of Kir6.2/SUR1 channel expression leads to damaging of cells116, thereby partially forwarding towards apoptosis. Studies have shown that in the mid part of SN DA nerves associated with DMS, K-ATP channels promote changeover from sustained response due to tonicity to NMDAR mediated bursting within itself. KATP increases the Ca2+ concentration in nerves connected to NMDA receptor molecules117, thereby increasing the ROS generation which influences neuronal stimulatory action, further damaging thousands of neurons and causing induction of PD118. Removal of Kir6.2 promotes the accession of DA phenotypes from nuclear-receptor-related 1 precursor, inhibits a decrease in glial cell-line-derived neurotrophic-factor (NF), promotes cellular distinctions in the nerves of the midbrain, which harms microRNA133b concentration119, and inhibits an increase in Fe2+ concentration in the mesencephalon of MPTP treated mouse, thereby suppressing the regulation of IRP-IRE lighter chain models and hence keeping SN DA nerve cells protected against MPTP-attack120. Hence, it can be said that Kir6.2 has a vast role to play in the regulation of KATP/Kir6.2 channels and in the diagnosis of PD.
Role of a type K-1 channels
Kv-4 generated type AK+ currents which included tetrameric subunits with Hexa-transmembrane domains (S1–S4 and S5-S6) (Fig. 9). Among them, S5 and S6 domains are of type A subunit formed-loops in the Kv-4 channel, which selectively allow permeability of ions. S1, S2, S3, and S4 of type A subunits formed voltage sensor domains121. Auxiliary K-Chips co-assembled with the P-looped performers in Kv-4 subunits which forms a complex of K+ channels, which helped in the regulation, expressions, and gate-like characteristics of Kv-4 current. The Kv-4 contains three different genetic materials from Kv4.1–4.3. Kv4.2 and 4.3 express well in the mesencephalon of dopaminergic nerve cells122. Interaction of Kv4.3 with K-Chip3, generates type AK+ currents, which inactivates the components of the extracellular K+ currents located in SNc dopaminergic nerves. Type AK+ channels in dopaminergic nerves of SN contributed to pacemaker-controlling tonic activities81. Inhibitory action of type-AK+ channel depolarized and increased the excitation by changing over from tonic-firing to burst-firing, thereby influencing dopamine-release123. MSN integrates various signaling pathways inside the cell thus causing dynamic modulation of membrane excitation. Intrastriatal infusion of AmmTX-3 reduced the deficiency of motor neurons, reduced anxiousness, and restored memorization power in 6-OHDA treated rodents. Also, 4-AMP inhibited the type-AK+ channel, which decreased apomorphine-induced rotational-test (RT) in 6-OHDA-induced rodents. The glia-derived neurotrophic factor (NF), regulated developmental and functional parts of the CNS. Type-AK+ channel in mesencephalon dopaminergic nerve cells triggered mitogen-activated kinases, thereby suggesting type-AK+ channels to be a promising diagnostic measure in Parkinsonism82.
Fig. 9. Mechanistic view of roles of different K+-channels in the diagnosis and treatment of the PD pathogenesis.
Cellular and animal models of Parkinsonism showed that SK channels, Type-A K+ channels, and Kv-7/ KCNQ channels possess a potential for PD diagnosis. Activators and blockers of these three potassium channels protect the dopaminergic nerve cells in SNc, modulate excitation of nerve cells, influence the release of DA, and attenuate motor symptoms. NMDA N-methyl-D-aspartate receptor, NADPH Nicotinamide adenine dinucleotide phosphate oxidase, MAPK Mitogen-Activated Protein Kinase pathway, GDNF Glial-cell derived neurotrophic factor.
Role of SK channels
SK channel regulates dopaminergic neuron functioning and excites the nerves of the basal ganglion. SN dopaminergic nerves are activated by these channels on induction of a single action potential by the offset of hyperpolarizing stimulus, reduction of a neuron’s firing rate, and maintaining the intrinsic current automaticity124–126. The boundary of the SK channel promotes N-methyl-D-aspartate-induced abnormal bursting in dopaminergic nerves, thereby activating Ca2+ dependent cellular activities and enhancing dopaminergic release in the corpus striatum, thus relieving PD symptoms. SK channel activation leads to neurotoxin-induced dopaminergic apoptosis (Table 1)127.
Role of Voltage-Gated Ca2+ channels
Voltage gated calcium channels (CaV) are responsible for exaggerating PD and blockade of the midbrain. However, when specific binders/blockers for each Cav isoform are absent, the exact role of either Cav1.2 or Cav1.3 in degeneration or protection of neurons is very difficult to understand. To decipher this mystry, CaV gene KO animal models were useful indicating that dihydropyridines have diverse effects on different types of these channel isoforms. Due to its exemplary roles in SN dopamine neurons and diverse spread across the brain CaV channels can be a potential drug target for PD, also to monitor the progress of any related treatment efficacy thereby reducing the side-effects of available therapeutics, if any (Fig. 8).
The link between α-synuclein-GBA-LRRK2 and ion-channels/gap-junctions to develop PD
It has been discussed above in the introduction that there are seven known causation alleles for familial PD which include α-synuclein, GBA, and LRRK24,5. Along with this α-synuclein-GBA-LRRK2 axis, within the complex neural network9, gap-junction-based synaptic vesicles remain scattered, thereby playing an important function in the developmental processes of the CNS and PD pathology. Moreover, gap-junctions and ion-channels both facilitate cell-cell interaction as well as the exchange of ions and tiny signaling chemicals9. They also act as a linker between the nerve cells and glia, namely astrocytes, microglia, and oligodendrocytes. Therefore, many structural or functional alterations in these junctions and ion-channels may lead to PD pathogenesis facilitated by α-synuclein-GBA-LRRK2 axis10.
LRRK2 contains a Ras of complex (ROC) GTPase domain, C-terminal of ROC (COR) linker region, and serine/threonine kinase domain, N-terminal ankyrin domain, a leucine-rich repeat (LRR), and a C-terminal WD40 domain128,129. All these domains are responsible for diverse array of protein-protein interactions. As many as eight pathologic LRRK2 mutations (G2019S, R1441G/H/C, I2012T, Y1699C, I2020T, and N1437H) are linked with PD94,130,131. LRRK2 can get self-phosphorylated with the help of a P-loop with a DFG (conserved residues Asp–Phe–Gly)-APE motif, thereby regulating its functionality as a kinase132,133. α-synuclein directly interacts with LRRK2 thereby they both help to regulate each others’ functionalities (Fig. 10). α-synuclein can also aggregate thereby forming oligomers which then form fibrils to generate LB during physiological or other stress134,135. S129 of almost 90% of α-synuclein in LB gets phosphorylated in PD patients136,137. Since LRRK2 is a serine-threonine kinase, its hyperactive mutant G2019S LRRK2 can phosphorylate α-synuclein, thereby aggregating the latter and promoting apoptosis which is reversed by the inhibitors of LRRK2138–142. In contrast to this, there are reports that in vivo (at least in mice model), α-synuclein is not a substrate for LRRK2 kinase143,144. Moreover, a deletion-based double transgenic animal study has indicated that the LRRK2 kinase is not responsible for promoting A53T α-synuclein-driven neuropathophysiology143. At the same time, many other kinases like G-protein coupled receptor kinases, casein kinases, and polo-like kinases, are all responsible for phosphorylating α-synuclein145–149. LRRK2 and α-synuclein have been co-immunoprecipitated from transfected HEK293 cells under oxidative stress141, from brain tissue extracts of human PD and dementia with Lewy body (DLB) patients, but not from age-matched control brains140,141. All these effects were confirmed with the help of studies in transgenic mice, primary neuron-expressing mutant G2019S LRRK2150,151, and in human induced pluripotent stem cell (iPSC)-derived neurons from G2019S LRRK2 carriers151. While these research reports conceptualized the effect of LRRK2 in α-synuclein-mediated cellular toxicity and a link between themselves, their exact mechanism of interaction is unclear to date. However, chaperones have been experimentally proven to be expected intermediates between LRRK2 and α-synuclein.
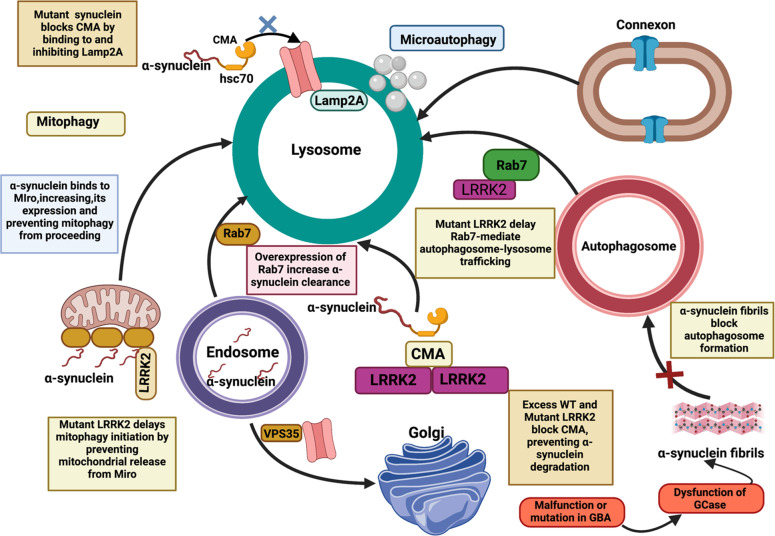
Fig. 10. Cross connection between α-synuclein-GBA-LRRK2 axis and ion-channels/gap-junctions to develop PD.
This is an example of convergent processes between LRRK2, GBA, and α-synuclein, with both impacting the autophagy-lysosomal system but acting on different targets within the system. CMA and autophagosome formation can be blocked by mutant or aggregated α-synuclein, and mutant LRRK2 can likewise impede CMA, impair mitophagy, and delay autophagosome trafficking. Malfunction in GBA can lead to the production of less active or inactive GCase thereby leading to aggregation of α-synuclein. This LRRK2-GBA-α-synuclein axis can also interfere with diverse neuronal ion-channels and gap-jucntions to PD pathogenesis. CMA Chaperone-mediated autophagy, Lamp2a lysosome-associated membrane protein type 2a, GCase β-glucocerebrosidase, GBA gene encoding GCase.
LRRK2 is also responsible for lysosomal protein trafficking and morphology. For example, PD patients’ fibroblasts carrying its related mutations resulted in dysregulated lysosomal morphology due to an enhanced influx of Ca2+ into the lysosome with the help of a diverse array of associated ion-channels152 (Fig. 10). Influx of this calcium can be reduced with the help of inhibitors of the two-pore calcium channels that are expressed over all acidic vesicles including endosomes and lysosomes of the E-L system153,154. Knocking out of LRRK2 in mice model resulted in deregulated clearance of proteins and over-accumulation of α-synuclein, thereby increasing the apoptotic cell death and oxidative impairments155. Overexpressed G2019S LRRK2 could reduce degradation capacity and enlarged lysosomes in astrocytes due to enhanced kinase activity of LRRK2156. Similar was the case in a study involving primary neurons cultured from G2019S LRRK2 knock-in mice which was diminished by inhibitors of LRRK2156. As many as fourteen Rab family members have been discovered to be phosphorylated by LRRK2 in their switch-II domains which is proof of a link between LRRK2 and the E-L system concerning its involvement in PD157. Rab3a and Rab8a can aid vesicular trafficking from the ER to the Golgi apparatus, thereby reducing α-synuclein-dependent cytotoxicity in animal models of PD158. Rab7 on the other hand is involved in the maturation of the autophagosome and early-to-late endosomes158,159. In an experiment in Drosophila Rab7 was found to connect LRRK2 and α-synuclein, as the former enhanced the clearance of α-synuclein aggregates, decreases cellular death, and protected locomotor malfunctions160. Nuclear factor erythroid-2 related factor (Nrf2), being an antioxidant protein, can be a promising drug target for neuronal damages161. Activation of Nrf2 reduces toxicity associated with LRRK2 and α-synuclein by enhancing the accumulation of LRRK2 in inclusion bodies and subsequently decreases its activity in other parts of the neuron162. It emphasizes that mutant LRRK2 may function as a negative regulator of autophagy thereby leading to α-synuclein deposition in DA neurons. α-synuclein can dysregulate E-L system thereby accelerating α-synuclein deposition using a feedback mechanism.
Gaucher disease occurs due to the loss of function of the lysosomal enzyme β-glucocerebrosidase (GCase), and the consequently increased level of glucosylceramide163,164. Such patients and carriers of heterozygous mutations in GBA (gene encoding GCase), are at higher threat to develop PD165–168. It is also now evident that accumulation of glycosphingolipids due to dysfunction of GCase can also enhance deposition of α-synuclein169. Such experiments were done in primary cultures of human iPSC170, and in rat brain171. Downregulation of GCase is further linked with reduced production of Beclin 1 (involved in autophagy). This was controlled via inactivating protein phosphatase 2A171. Combining all these findings, it can be postulated that a reduction in lysosomal enzymes can initiate a feedback mechanism by which inhibition of autophagy can be achieved thereby inhibiting upstream lysosomal functions. There is a controversy between the increase and decrease in GCase activity due to LRRK2 mutations, respectively172,173. It indicates that G2019S LRRK2 mutations are responsible for different pathological pathways. Hyperactivation of GCase triggers removal of α-synuclein and reversal of lysosomal activities in iPSC-derived human midbrain DA neurons from patients with GBA-related PD or idiopathic PD174. Downregulation of LRRK2 enzyme activity was seen to hyperactivate GCase in DA neurons obtained from patients with either LRRK2 or GBA mutations, thereby decreasing the piling up of α-synuclein in such nerves172. Hyperactivation of LRRK2 can also result in Y307 phosphorylation and inactivation of Rab10, which in turn leads to a reduction in GCase activity. The latter can be prevented with inhibitors of LRRK2. Results showing that α-synuclein can hinder the lysosomal activity of GCase in neurons and brain tissue of idiopathic PD patients170 in turn convinced that the lysosome is a convergent organelle where dysfunctional LRRK2 and/or α-synuclein have destructive effects on lysosome function. The latter results in neurodegeneration like PD. Dysregualtion of this LRRK2-α-synuclein-GBA axis can also justify the increased tendency of DA neurons, since VPS35 and LRRK2 are crucial for vesicular trafficking and deregulation in these proteins may result into mistargeting of the dopamine transporter, DAT, thereby abnormal dopamine metabolism and oxidation (Fig. 10)175. The later in turn is reported to downregulate GCase176 and thus indicating the possibility of a negative feedback loop, making DA neurons uniquely vulnerable to degeneration.
How do α-synuclein/GBA/LRRK2 interfere with the function of ion-channels/gap-junctions thereby leading to PD pathogenesis?
LRRK2 controls Ca2+ influx via controlling the voltage-gated Ca2+ (CaV) channels. Presynaptic CaV2.1 channels in turn stimulate neurotransmitter release. Like LRRK2, many other protein kinases, G-proteins, and Ca2+ binding proteins are also key regulators of CaV2.1. Recently, Cade Bedford et al.177 discovered that LRRK2 protein interacts with specific synaptic proteins and influences synapsis. As synaptic proteins interact functionally with CaV2.1 channels, synaptic transmission was seen to be triggered by Ca2+ entry via CaV2.1. CaV2.1 channel properties were measured using a whole-cell patch-clamp in HEK293 cells transfected with CaV2.1 subunits and various LRRK2 constructs. Cade Bedford et al.177 results demonstrated that both WT and G2019S LRRK2 mutants result in a significant enhancement in whole-cell Ca2+ current in comparison to cells expressing only CaV2.1 channel. Moreover, LRRK2 expression resulted in a significant hyperpolarizing shift in voltage-dependent activation while having no significant effect on inactivation properties. These CaV2.1 functional alternations are probably due to LRRK2 since co-immuno-precipitation of LRRK2 and the β3 CaV channel was observed by scientists177. Moreover, CaV2.1 channel activities are dependent on LRRK2 as evident from reversing effect by use of LRRK2 inhibitors. Interestingly, LRRK2 also augmented endogenous voltage-gated Ca2+ channel function in PC12 cells suggesting other CaV channels could also be regulated by LRRK2. Overall, these findings support a novel physiological role for LRRK2 in regulating CaV2.1 function that could have implications for how mutations in LRRK2 contribute to PD pathophysiology. Moreover, overexpression of Kv2.1 channel can result in the reduction of DA in the striatum terminals of DA neurons and SN84. They can reduce K+ level thereby stimulating enzymes like nucleases and proteases intracellularly, leading to cell death85. This in turn also increases the intracellular Ca2+ concentration thereby increasing the Ca2+ influx into the cell, leading to cell damage and thereby linking with the flow of K+ inside the cell, hence causing PD86. Also, constitutive binding of calmodulin to the closer proximity of the C-terminus detects the action of Ca2+. LRKK2 mediated phosphorylated calmodulin influences susceptibility of SK channel. Calmodulin after binding to Ca2+, activates SK channels leading to a change in the membrane potential of a cell, thereby reducing its excitation105(Fig. 8). KATP channels also increase the Ca2+ concentration in nerves connected to NMDA receptor molecules117, thereby increasing the ROS generation which influences neuronal stimulatory action, increasing the amount of α-synuclein thereby further damaging thousands of neurons and causing induction of PD118. α-synuclein, is a type of KATP channel with having presynaptic neurons, functions informing and maintaining synapses178,179. α-synuclein present outside the cell membrane may be transferred from injuries to normal nerve cells by the prion-like mechanistic pathway, and this cellular transduction was observed in PD affected brain. In the striatum of mice, the KATP channel was activated by γ-aminobutyric nerve cells to reduce γ-aminobutyric acid concentration, further decreasing GABA receptor transmission on glutamate/aspartate neurons. Therefore, the Ca2+ level increases and triggers the α-synuclein secretory mechanism. Increased level of SUR1-mRNA in α-synuclein transfected MES23.5 cells and selectively activating Kir6.2/SUR1 in the SN leads to degenerated DA nerve cells; which proves that SUR1 plays a promising role in PD180. KATP/α-synuclein channel is expressed in the islet-β cell for controlling the secretion of insulin, thus interacting with Kir6.2 in ISGs to reduce the level of insulin. Misfolding in α-synuclein causes T2DM and PD by spreading prion-like activity and apoptosis181. Kir6.2-antisense oligo-DNAs were administered into GP, for the case of 6−OHDA hemiparkinsonian rat, which reduced the expressibility of Kir6.2-mRNA, thereby reducing apomorphine-induced the other side turns in the models. Pre-treatment of rodents with 6−OHDA induced abnormalities in PD with glibenclamide shows improvements in severe behavioral-symptoms182. Use of NaHS reduced 6−OHDA related behavioral symptoms and cellular damage of DA cells in the SN183. According to the clinical trials database, disease-modifying drugs namely antioxidants, botanicals, cell replacement therapies, mitochondrial function enhancers, GBA modifiers, glucagon-like peptide-1 agonists, immunotherapy, kinase inhibitors, neurotrophic factors184, are under clinical trials for the treatment of PD.
Nevertheless, LRRK2, α-synuclein, and GBA, all target the gap-junctions and ion-channels of the CNS to produce PD pathogenesis which of course needs further attention to understand the underlying mechanism. This might be an innovative way of therapeutic strategy to treat PD patients.
Therapeutic interventions for PD involving ion-channels and gap-junctions
To explore PD treatments, it is indeed necessary to understand the varied gradients of advancement in PD sufferers, which indicate the medical (and pathologic) diversity of the illness. A growing knowledge of the etiology and pathogenesis of PD has evolved the theories regarding novel neuroprotective treatments which can be effectively implemented in the prodromal stage. Various drugs that are employed in the treatment of PD are tabulated in Table 2 and shown pictorially in Figs. 9 and 11. Additionally, cell-replacement therapies were found to be advantageous over fetal cell-derived therapies185, in developmental and stem cell biology that comprises DA neurons derived from human pluripotent stem cells. However, improper knowledge about the treatment of PD, choice of inaccurate animal models and unvalidated biomarkers, and limitations in trial design restrict the development of effective neuroprotective therapies for PD. Novel transgenic animal models, adaptive and delayed-start trial designs, and identification of potential serum, cerebrospinal fluid, and neuroimaging biomarkers facilitates the development and testing of effective disease-modifying therapies (Table 3).
Table 2.
Drugs that are involved in the treatment of PD.
| Drugs | Mode of action | Side effects | Adverse effects | References |
|---|---|---|---|---|
| L-DOPA | DA protagonist | Increases DA levels | Short of breath, vomit, lower BP, tired, drowsy. | 214–216 |
| Selegiline | MAOB inhibitors | Maintaining Levo-DOPA | Dizzy, dried mouth, insomnia, muscular pains, rashes, shortness of breath, constipation, headaches, tachycardia, arrhythmia, hallucination | 217 |
| Creatine | Boosting mitochondria | Antioxidants, prevents MPTP induced apoptosis | Short of breath, stomachpain, diarrhea, muscular cramp, swell in specific body parts, weight gain. | 218 |
| Bromocriptine, Apomorphine | DA protagonist | Increase DA concentrations | Drowsy, short of breath, vomit, dried mouth, dizzy, swelling in limbs, fainting, decrease in BP, confusions, hallucination | 219 |
| Pramipexole, Ropinirole | Non-ergoline dopamine agonist, specific to D2 receptors | Peripheral and central dopaminergic stimulation | Dizziness, headache, nausea, and somnolence | 220 |
|
Entacapone and tolcapone |
Prevents DA degradation | Extends Levo-DOPA effect | Short of breath, diarrhea, orthostatic hypotension, urinaryproblems, dizziness, abnormal functioning of mitochondria | 221 |
| Amantadine | Activates DAsynthesisation | Increase DA concentrations | Confusions, urinary problems, feeling dizzy, fainting, problems in the sense organs, swelling in the limbs | 222 |
| Rofecoxib | COX2 inhibitor | Prevent inflammation | Spinal pain, diarrhea, dizziness, migraines, lack of energy, running nose, swelling in limbs, blur in eyesight, intestinal problems. | 223 |
|
Pimavanserin (ACP-103) |
Blockage of Serotonin receptor | Decreases L-DOPA related problems | Hyperprolactinemia, improper functioning of menstrual and sex organs, akathisia, distressful motor disturbance, tiring body | 224 |
| Quetiapine | Blockage of 5-HT2 and D2 receptor | Reduces agitated psychotic symptoms | Agitation, dizziness, tremors, anxiousness, hypertonia, indigestion, involuntary movements, worries, hyperkinesia, increased-libido, abnormality in gait, myoclonus, apathy, ataxia, hemiplegia, aphasia, BS (buccoglossal syndrome) | 225 |
|
CoQ-10 (Ubiquinone) |
Improved functioning of mitochondria | Antioxidants lower disease progression in the earlystages | Low BP, hemorrhage, skin itchiness, shortness of breath, vomit, headaches, migraine, shortage of breath, spinal and chest pain, constipation, coughs, diarrhea, dizziness, faints, falls, fatigues, hearing problems, mild stroke, indigestion, insomnia, muscularpain, night-sweat, pharyngeal infection | 226,227 |
|
Entacapone, tolcapone |
COMT inhibitor | Inhibit dopamine degradation | Diarrhea, shortness of breath, sleep disturbances, dizziness, urination disorders, pain in the abdomen, lower BP, hallucination | 228 |
|
S-Adenosylmethionine (SAM) |
Phospholipid-methylation and increases neural-communication | Improve DA transmissibility, decrease depression | GI diseases, GERD, and anxiousness | 229 |
| Carbenoxolone | Inhibitors of Gap junctions Cx38, Cx26, Cx43, Panx1 particularly | Disruption in electrolyte balance such as sodium retention and hypokalemia | Cardiac arrest left ventricle failure, visual impairments | 49,230 |
| Tonabersat | Inhibts connexin hemichannels | Discomfort and drowsiness | Severe adverse effects appeared infrequent, as well as none individual dropped out of any trial due to them | 231 |
| ketamine, propofol and dexmedetomidine | In vivo, tight junctions interaction with astrocytes is considerably blocked. | Dissociation elevated blood pressure | None of the drugs include adverse side effects | 188,232 |
| Gastrodin | It current plays an inhibitory action with the expression of Cx43 and also to some extent the activity of hemichannels | Drugs is having very less side effects. | The drug includes no adverse side effects. | 233 |
| Cannabinoids (CB’s) | In neuro-inflammatory circumstances, CBs are critical chemicals to prevent excessive activation of connexin hemichannels and pannexin channels. | Obesity, elevated level of calorie intake | Impaired cognition | 234 |
| Apamin | Inhibits SK channels | Not available | Not available | 235 |
| Isradipine | Targets LTCCs | Increased dosages might exacerbate trembling, induce disorientation, even induce vomiting, alterations in blood, tension | Same as the side effects mentioned | 236 |
| Safinamide | Inhibits Ca2+ channel activity | Elevated increase of high blood pressure | Dyskinesia, Dyspepsia, Orthostatic hypotension | 235 |
| 4-Aminopyridine | Blocks Kv channels | Not available | Not available | 235 |
Fig. 11. Pictorial depiction of different blockers of ion-channels and gap-jucntions to treat PD.
While DHPs and Safinamide block Ca2+ and Na+ channels, respectively, Amantadine blocks NMDA receptor to treat PD. Similarly, Carbenoxolone (CBX), Octanol, and Gastrodin can block Cx26, Cx38, and Cx43, respectively as a treatment for PD. Trodusquemine can prevent aggregation of α-synuclein and related mutations to prevent Parkinsonism. DHPS Dihydropyridines (DHPs), ROS Reactive Oxygen Species.
Table 3.
Latest technologies used for detecting and neuroimaging PD.
| Technologies | Applications in PD | References |
|---|---|---|
| Cueing Devices | PD patients receives signals to modify their motions in right directions. Patients receive sensory feedback from vibrating sensors and/or electric induder. | 237 |
| Biopotential Devices | A biopotential device is integrated into a wearable device like EEG, ECG, and magnetoencephalogram for the treatment and monitoring of PD patients. | 237 |
| Optical Motion Tracker | This helps to monitor the movements of the POD patients with the help of a radio frequency and/or optical device. | 237 |
| Audio Recording | PD patients’ speech and swallowing like indications are recorded using a mobile phone or a voice recorder for diagnosis and prognosis purposes. | 237 |
| Video Recording | Recors gait and motor issues in PD patients. | 237 |
| Force/Pressure | Force/pressure sensing devices can be inserted into gait mats to monitor the gait quality of a PD patient. | 237 |
| Smart phone | A smart phone screen can record handwriting, its microphone can record speech, and the camera can record PD patient’s motions. | 237 |
| X-ray computed tomography and brain MRI | It can diagnose structural lesions present with vascular pathophysiology/neoplasms and detects the amount and location of brain-atrophy. | 238 |
| PET or SPECT | It can provide images for DA related activities in the presynaptic neurons to diagnose and monitor PD. | 238 |
Mechanism of action of blockers of gap-junctions to treat PD
A wide range of substances has been documented to work as gap-junction blockers (GJBs). Nevertheless, those chemicals are often rarely specific and can also potentially adhere to a wide range of different sites, resulting in multiple phenotypic expression side-effects. A variety of GJBs was developed for use in PD49. GJBs such as carbenoxolone (CBX) and octanol strongly suppress the activation of Cx38- and Cx26-based tight junctions. CBX was also demonstrated to inhibit α-synuclein aggregation inside a rotenone-induced PD mouse (Fig. 11)186. This result is analogous to the suppression of inflammatory response and oxidative damage caused by α-synuclein accumulation, which is attributable in addition to inhibitory actions of CBX on Cx43 tight-junctions187. Tonabersat is a cis-benzopyran chemical that blocks connexin hemichannels. Tonabersat has been shown to lower Cx26 activity inside the cortex by blocking the p38-mitogen triggered protein kinase pathway. Additionally, it can effectively attach to and inhibit Cx43 hemichannels49. In vitro, anesthetics such as ketamine, propofol, and dexmedetomidine dramatically inhibit tight-junctions interaction amongst astrocytes188. Gastrodin, a Chinese medicinal herb component, was also utilized as a GJB. In rotenone-induced PD rodent models, gastrodin inhibits Cx43 synthesis, activation, and cellular interaction. Interestingly, gastrodin inhibits the symptomatic characteristics of PD inside this animal paradigm189. The lowered expression of Cx43 by gastrodin may affect Cx43 hemichannel activity, which might explain the desirable outcomes190. Cannabinoids (CBs) have been considered as possible treatments for PD. CBS also has beneficial effects on neural tissues through altering gap-junctional signaling. Anandamide, for example, which is an endogenous arachidonic acid derivate, can activate neuronal CB receptors by suppressing gap-junctional conductivity throughout astrocytes49. According to the clinical trials database, disease-modifying drugs namely antioxidants, botanicals, cell replacement therapies, mitochondrial function enhancers, GBA modifiers, glucagon-like peptide-1 agonists, immunotherapy, kinase inhibitors, neurotrophic factors184, are under clinical trials. Dizocilpine, 4-AP, has shown promising results in the pre-clinical studies and needs further investigation. Drugs such as Safinamide191, Zonisamide192, Amantadine, etc. have opened up the scope for further exploration of ion-channels for slowing down the disease progression193.
Mechanism of actions of blockers of ion-channels to cause PD
However, the difficulty with ion-channels as a target is due to their wide expression in the brain, hence activation or inhibition of the ion-channels might lead to many certain side effects if not specific. Therefore, drugs and small molecules need to be developed in a very selective manner. This is where comes the necessity of crossing the blood-brain barrier (BBB). Although direct injection of drugs in the required brain regions, was found to be extremely invasive and expensive, it was found to be a promising alternative. In this regard, assisted transport of drugs through BBB was performed using specialized nanoparticles or receptors via endocytosis or transcytosis194. Several studies have shown the effectiveness of using nanoparticles for delivering drugs specifically to dopaminergic neurons in the animal models of PD. In use of lipid nanoparticles covered with lactoferrin to deliver GDNF in 6−OHDA lesioned rats195, were shown to improve the motor phenotype and TH immunostaining195. In the same manner, shRNA against the SNCA gene has been delivered to MPTP-treated mice using Fe3O4 nanoparticles. These magnetic nanoparticles used NGF to bind the tyrosine kinase receptors expressed specifically on neurons for binding and prevented MPTP-mediated neurodegeneration196,197. A very recent study reported the enhanced expression of CACNA1D, a gene encoding a subunit of the voltage-gated calcium channels, in PD microarray data197. Therefore, there is a ray of hope that shortly ion-channel subunits might serve as useful biomarkers and targets for the treatment of PD as shown in Fig. 11.
Apart from the two major modes of action of the ion-channels, i.e., (1) pore plugging, and (2) allosteric binding, there are other mechanisms by which inhibition of ion-channels occurs. In the pore plugging mode of action, there is a physical obstruction in the transport of ions through the channel pore via the binding of inhibitors in the pore region. However, this method of inhibition is a reversible process, and the normal functionality of this channel can be restored after washing out the inhibitor. Non-allosteric binding of STX and TTX plug the NaV channel from the extracellular part of the pore. At times when the inhibitor passes first through the cell membrane, plugging occurs from the intracellular side of the channel. TeA (Tenuazonic acid) and QA (Quaternary ammonium) ions inhibit the KV channels from the intracellular side, by open channel inhibition, which can take place only when the channel is in an open state and not when closed. Depolarization of the cell membrane opens up the voltage gate for TeA to enter the central cavity and become lodged to plug the channel.
On the other hand, the allosteric mode of binding requires a specific binding site on the extracellular part of the pore where an allosteric inhibitor binds to the channel, thereby causing conformational changes, hence preventing the normal functionality of the channel. This mechanism can be relatable to the way agonist and antagonist bind to the receptors of the outer membrane, and similar to the way nAChR undergoes ligand-binding. The allosteric inhibitor binds to and blocks the channel in a nonconducting conformational state. Dihydropyridines (DHPs), L-type Ca2+ channel (CaV1) inhibitors, and most natural toxins use this method of channel inhibition. In the case of voltage-gated channels, it is often located somewhere on the voltage-sensor domain (S4) or the inactivation ball (Fig. 11). The voltage-sensor domain is partially exposed to the extracellular space, which makes the binding easier. Such modulators interfere with the voltage-sensor and thus modulate the ion-channel gating. For this reason, they are often referred to as voltage-sensor modulators or gating modifiers. Gating modifiers have a favorite binding site for inhibitors and channel modulators located on the voltage-sensor domain (S4), thereby leading to a channel activation-inhibition mechanism, or even an increased, delayed, or complete inactivation process.
To summarize, ion-channels play a vital role in driving PD pathology. There is numerous emerging evidence that proves the usefulness of ion-channels as a therapeutic target for PD. However, to effectively utilize ion-channel modulation for therapies, it is important to deeply understand the alteration of ionic homeostasis with the disease course. Simultaneously, there is a need to understand the interlinked pathways which regulate the expression and functioning of the various channels that will pave the way for the development of therapeutics to bring about the much-needed relief to PD patients.
Conclusion and future perspectives
PD has afflicted people over many generations and is a debilitating neurodegenerative disorder. To date, much of the research has been focused on unraveling the molecular details of α-synuclein and tau proteins. It is an urgent need to look into various systemic aspects of the disease. In this current review article, we have shed light on new areas of research within PD. The importance of neural cell-junctions and ion-channels may open up new avenues to better diagnose and treat PD. Through a thorough literature survey, we put forward several burning questions for the better management of this disease. Mimicking mammalian Parkinsonism is an important aspect of this and certain techniques and methodologies need to be developed38, especially for proper understanding of this complicated ailment. Some of these are: (i) Developing different models of mammals which could be a combination of many modular organisms for genetic and toxin effects; (ii) The use of non-dopamine-drugs, namely α2-adrenergic protagonists, adenosine-A2a protagonists, is needed for PD diagnosis; (iii) Usage of IPX-066, XP-21279, and Opicapone, MAO inhibitors such as safinamide to reduce depression and dyskinesia; (iv) Targets ALP-technique and UPS-technique in PD treatments; (v) Developing transplantation therapy using dopamine nerves from ISCs (Interval Specific Congenic Strains); (vi) miRNA/siRNA based approaches for the inhibition of mRNA of abnormal folding in protein complexes; (vii) Using CRISP-Cas9 technique in correcting mutant-genes198; (viii) To understand if PD shows a dual disorder in animals and humans; (ix) Using electrophysiology experiments to further understand PD; (x) Development of different ion-channels to deal with neuroinflammation during Parkinsonism, etc.
Nevertheless, several issues need to be addressed before effective gene therapy can be safely used to treat PD. Major efforts are needed in identifying genetic materials for rectifying, developing safe-maker genes, and modulation of compact expressibility of genes in the central nervous system199. Increased barrier-permeability in gut-lumen allows reduction of negatively regulated brain neurons and neurons of the GI tract, thus providing a diagnostic pathway in Parkinsonism and GI-related diseases198. Novel exosome-based diagnostic methodology from patients’ biofluids has shown the potential to diagnose various neurodegenerative diseases including Parkinson’s200,201 and Alzheimer’s disease201. An in vivo TST (triple sugar test), fecal-biomarkers, ex vivo PT, and tissue-staining techniques allowed for comparing and validating different techniques for pathological methods in the treatment of PD. The reductionist-model system can help elucidate the pattern of processes and pros and cons of these methodologies, thus identifying microorganisms and MF (Microbial Factor), which could have promoted α-synuclein pathways in nerve cells. Patients with fibroblast-derived iPSC are another approach for future studies. iPS cells were divided into three-dimensional intestinal organoids, also known as mini-guts202. A three-dimensional epithelial culture may be essential for EPA (epithelial permeability analysis)202. iPSC allows cultures of intestinal organoids with ENS at the bottom of cells that were collected for controlled experiments. iPSC-derived intestinal organoids lacked epigenetics and displayed immaturities202 with lacking related aspects of Parkinsonism. From a different aspect, it can be said that the mini-gut models were utilized for comparing with the genes of the patient, which could have been one of the parameters contributing toward the integration of mucosal barriers, which is yet to be studied under PD.
Acknowledgements
SB is grateful to the University Grant Commission (UGC), Govt. of India for providing a non-NET fellowship for the current work. VS is grateful for support from the Department of Health, Education and Technology, Lulea University of Technology, Sweden. SKD acknowledges the Dr. B.R. Ambedkar Center for Biomedical Research (ACBR), University of Delhi, India for various help to complete the current work. SKD also acknowledges the Institution of Eminence grant (grant ID: IoE/2021/12/FRP) from the University of Delhi, India for providing financial support for the current work. All authors are thankful to reviewers for their constructive remarks on our work. SKD is also grateful to Profs. Suman Kundu, and DP Sarkar from the Department of Biochemistry and Prof. BK Thelma from the Department of Genetics of the University of Delhi South Campus, India for their continuous support and scientific discussions to improve the current manuscript. Figures and schematics were created with BioRender.com.
Author contributions
S.K.D. and V.S. conceived the manuscript. S.P.C., S.B., and S.S. worked equally, hence are shared first authors. S.P.C., S.S., and S.K.D. wrote the first draft of the manuscript. S.B. prepared the initial figures and figure legends, while S.K.D. edited the same. All authors have proofread the manuscript. All authors have approved the manuscript. S.K. and F.N. provided important critical suggestions to improve the manuscript.
Funding
Open access funding provided by Lulea University of Technology.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Saptamita Paul Choudhury, Sarika Bano, Srijon Sen.
These authors jointly supervised this work: Saroj Kumar, Fredrik Nikolajeff.
Contributor Information
Sanjay Kumar Dey, Email: sdey@acbr.du.ac.in.
Vaibhav Sharma, Email: vaibhav.sharma@ltu.se.
References
- 1.Hirsch L, Jette N, Frolkis A, Steeves T, Pringsheim T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology. 2016;46:292–300. doi: 10.1159/000445751. [DOI] [PubMed] [Google Scholar]
- 2.Lill CM. Genetics of Parkinson’s disease. Mol. Cell. probes. 2016;30:386–396. doi: 10.1016/j.mcp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Ball N, Teo W-P, Chandra S, Chapman J. Parkinson’s Disease and the Environment. Front Neurol. 2019;10:218–218. doi: 10.3389/fneur.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verstraeten A, Theuns J, Van Broeckhoven C. Progress in unraveling the genetic etiology of Parkinson disease in a genomic era. Trends Genet.: TIG. 2015;31:140–149. doi: 10.1016/j.tig.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Kalinderi, K., Bostantjopoulou, S. & Fidani, L. The genetic background of Parkinson’s disease: current progress and future prospects. Acta. Neurol. Scand.134, 314–326 (2016). [DOI] [PubMed]
- 6.Desai BS, Monahan AJ, Carvey PM, Hendey B. Blood–brain barrier pathology in Alzheimer’s and Parkinson’s disease: implications for drug therapy. Cell Transplant. 2007;16:285–299. doi: 10.3727/000000007783464731. [DOI] [PubMed] [Google Scholar]
- 7.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 8.DeWeerdt S. How to map the brain. Nature. 2019;571:S6–s8. doi: 10.1038/d41586-019-02208-0. [DOI] [PubMed] [Google Scholar]
- 9.Dbouk HA, Mroue RM, El-Sabban ME, Talhouk RS. Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun. Signal. 2009;7:4–4. doi: 10.1186/1478-811X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blauwendraat C, Nalls MA, Singleton AB. The genetic architecture of Parkinson’s disease. Lancet Neurol. 2020;19:170–178. doi: 10.1016/S1474-4422(19)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT. Amyloid pores from pathogenic mutations. Nature. 2002;418:291–291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 12.Hazell AS, Itzhak Y, Liu H, Norenberg MD. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) decreases glutamate uptake in cultured astrocytes. J. neurochemistry. 1997;68:2216–2219. doi: 10.1046/j.1471-4159.1997.68052216.x. [DOI] [PubMed] [Google Scholar]
- 13.Loewenstein Y. A possible role of olivary gap-junctions in the generation of physiological and pathological tremors. Mol. psychiatry. 2002;7:129–129. doi: 10.1038/sj.mp.4000994. [DOI] [PubMed] [Google Scholar]
- 14.Elble RJ. Central mechanisms of tremor. J. Clin. Neurophysiol. 1996;13:133–144. doi: 10.1097/00004691-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Lampl I, Yarom Y. Subthreshold oscillations and resonant behavior: two manifestations of the same mechanism. Neuroscience. 1997;78:325–341. doi: 10.1016/S0306-4522(96)00588-X. [DOI] [PubMed] [Google Scholar]
- 16.Nakase T, Naus CCG. Gap junctions and neurological disorders of the central nervous system. Biochimica et. Biophysica Acta (BBA) - Biomembranes. 2004;1662:149–158. doi: 10.1016/j.bbamem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez, O. F. et al. Role of Connexins 30, 36, and 43 in Brain Tumors, Neurodegenerative Diseases, and Neuroprotection. Cells.9, 846 (2020). [DOI] [PMC free article] [PubMed]
- 18.Surmeier DJ, Schumacker PT. Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease. J. Biol. Chem. 2013;288:10736–10741. doi: 10.1074/jbc.R112.410530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang YH, et al. Gene-Environment Interaction in Parkinson’s Disease: Coffee, ADORA2A, and CYP1A2. Neuroepidemiology. 2016;47:192–200. doi: 10.1159/000450855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming SM. Mechanisms of Gene-Environment Interactions in Parkinson’s Disease. Curr. Environ. health Rep. 2017;4:192–199. doi: 10.1007/s40572-017-0143-2. [DOI] [PubMed] [Google Scholar]
- 21.Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 22.Rietdijk, C. D., Perez-Pardo, P., Garssen, J., van Wezel, R. J. A. & Kraneveld, A. D. Exploring Braak’s Hypothesis of Parkinson’s Disease. Front. Neuro.8, 37 (2017). [DOI] [PMC free article] [PubMed]
- 23.Goldman JG, Postuma R. Premotor and nonmotor features of Parkinson’s disease. Curr. Opin. Neurol. 2014;27:434–441. doi: 10.1097/WCO.0000000000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moustafa AA, et al. Motor symptoms in Parkinson’s disease: A unified framework. Neurosci. Biobehav. Rev. 2016;68:727–740. doi: 10.1016/j.neubiorev.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Simon DK, Tanner CM, Brundin P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020;36:1–12. doi: 10.1016/j.cger.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flynn MR, Susi P. Neurological risks associated with manganese exposure from welding operations–a literature review. Int. J. Hyg. Environ. health. 2009;212:459–469. doi: 10.1016/j.ijheh.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15:1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 28.Magistrelli, L., Contaldi, E. & Comi, C. The Impact of SNCA Variations and Its Product Alpha-Synuclein on Non-Motor Features of Parkinson’s Disease. Life (Basel)11, 80804 (2021). [DOI] [PMC free article] [PubMed]
- 29.Meade RM, Fairlie DP, Mason JM. Alpha-synuclein structure and Parkinson’s disease – lessons and emerging principles. Mol. Neurodegeneration. 2019;14:29. doi: 10.1186/s13024-019-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cremades N, et al. Direct observation of the interconversion of normal and toxic forms of α-synuclein. Cell. 2012;149:1048–1059. doi: 10.1016/j.cell.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jankovic, J. & Tan, E. K. Parkinson’s disease: etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry. 91, 795–808 (2020). [DOI] [PubMed]
- 32.Rocha, E. M., De Miranda, B. & Sanders, L. H. Alpha-synuclein: pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 109, 249–257 (2018). [DOI] [PubMed]
- 33.Weng M, et al. The Sources of Reactive Oxygen Species and Its Possible Role in the Pathogenesis of Parkinson’s Disease. Parkinson#x2019;s. Dis. 2018;2018:9163040. doi: 10.1155/2018/9163040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabiei Z, Solati K, Amini-Khoei H. Phytotherapy in treatment of Parkinson’s disease: a review. Pharm. Biol. 2019;57:355–362. doi: 10.1080/13880209.2019.1618344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuzuhara S, Mori H, Izumiyama N, Yoshimura M, Ihara Y. Lewy bodies are ubiquitinated. Acta Neuropathologica. 1988;75:345–353. doi: 10.1007/BF00687787. [DOI] [PubMed] [Google Scholar]
- 36.McNaught KSP, Jenner P. Proteasomal function is impaired in substantia nigra in Parkinson’s disease. Neurosci. Lett. 2001;297:191–194. doi: 10.1016/S0304-3940(00)01701-8. [DOI] [PubMed] [Google Scholar]
- 37.Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 38.Maiti P, Manna J, Dunbar GL. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl. Neurodegeneration. 2017;6:28. doi: 10.1186/s40035-017-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Sci. (N. Y., N. Y.) 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 40.Haik KL, et al. 7-nitroindazole attenuates 6-hydroxydopamine-induced spatial learning deficits and dopamine neuron loss in a presymptomatic animal model of Parkinson’s disease. Exp. Clin. Psychopharmacol. 2008;16:178–189. doi: 10.1037/1064-1297.16.2.178. [DOI] [PubMed] [Google Scholar]
- 41.Yarwood, R., Hellicar, J., Woodman, P. G. & Lowe, M. Membrane trafficking in health and disease. Dis. Models Mech.13, 43448 (2020). [DOI] [PMC free article] [PubMed]
- 42.Abeliovich A, Gitler AD. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature. 2016;539:207–216. doi: 10.1038/nature20414. [DOI] [PubMed] [Google Scholar]
- 43.Nalls MA, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 2014;46:989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steger M, et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. eLife. 2016;5:e12813. doi: 10.7554/eLife.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banerjee R, Starkov AA, Beal MF, Thomas B. Mitochondrial dysfunction in the limelight of Parkinson’s disease pathogenesis. Biochimica et. Biophysica Acta (BBA) - Mol. Basis Dis. 2009;1792:651–663. doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhat AH, et al. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomedicine Pharmacother. = Biomedecine pharmacotherapie. 2015;74:101–110. doi: 10.1016/j.biopha.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 47.Breen DP, Halliday GM, Lang AE. Gut-brain axis and the spread of α-synuclein pathology: Vagal highway or dead end? Mov. Disord.: Off. J. Mov. Disord. Soc. 2019;34:307–316. doi: 10.1002/mds.27556. [DOI] [PubMed] [Google Scholar]
- 48.Sampson TR, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167:1469–1480.e1412. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmadian E, Eftekhari A, Samiei M, Maleki Dizaj S, Vinken M. The role and therapeutic potential of connexins, pannexins and their channels in Parkinson’s disease. Cell. Signal. 2019;58:111–118. doi: 10.1016/j.cellsig.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Vuyst, E. D. et al. Connexin Hemichannels and Gap Junction Channels Are Differentially Influenced by Lipopolysaccharide and Basic Fibroblast Growth Factor. Mol. Biol. Cell18, 34–46, (2007). [DOI] [PMC free article] [PubMed]
- 51.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiological Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 52.Belousov AB, Fontes JD, Freitas-Andrade M, Naus CC. Gap junctions and hemichannels: communicating cell death in neurodevelopment and disease. BMC Cell Biol. 2017;18:4. doi: 10.1186/s12860-016-0120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rash JE, et al. Immunogold evidence that neuronal gap junctions in adult rat brain and spinal cord contain connexin-36 but not connexin-32 or connexin-43. Proc. Natl Acad. Sci. USA. 2000;97:7573–7578. doi: 10.1073/pnas.97.13.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srinivas M, et al. Functional Properties of Channels Formed by the Neuronal Gap Junction Protein Connexin36. J. Neurosci. 1999;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giaume C, et al. Gap junctions in cultured astrocytes: single-channel currents and characterization of channel-forming protein. Neuron. 1991;6:133–143. doi: 10.1016/0896-6273(91)90128-M. [DOI] [PubMed] [Google Scholar]
- 56.Dermietzel R, Hertberg EL, Kessler JA, Spray DC. Gap junctions between cultured astrocytes: immunocytochemical, molecular, and electrophysiological analysis. J. Neurosci. 1991;11:1421–1432. doi: 10.1523/JNEUROSCI.11-05-01421.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dermietzel R, et al. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Brain Res Rev. 2000;32:45–56. doi: 10.1016/S0165-0173(99)00067-3. [DOI] [PubMed] [Google Scholar]
- 58.Batter DK, et al. Heterogeneity in gap junction expression in astrocytes cultured from different brain regions. Glia. 1992;6:213–221. doi: 10.1002/glia.440060309. [DOI] [PubMed] [Google Scholar]
- 59.Nagy JI, Rash JE. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Brain Res Rev. 2000;32:29–44. doi: 10.1016/S0165-0173(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 60.Mercier F, Hatton GI. Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: roles in stem cell proliferation and morphological plasticity? J. Comp. Neurol. 2001;431:88–104. doi: 10.1002/1096-9861(20010226)431:1<88::AID-CNE1057>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 61.Dermietzel R, et al. Oligodendrocytes express gap junction proteins connexin32 and connexin45. Glia. 1997;20:101–114. doi: 10.1002/(SICI)1098-1136(199706)20:2<101::AID-GLIA2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 62.Altevogt BM, Kleopa KA, Postma FR, Scherer SS, Paul DL. Connexin29 is uniquely distributed within myelinating glial cells of the central and peripheral nervous systems. J. Neurosci. 2002;22:6458–6470. doi: 10.1523/JNEUROSCI.22-15-06458.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang W, et al. Connexin29 is highly expressed in cochlear Schwann cells, and it is required for the normal development and function of the auditory nerve of mice. J. Neurosci. 2006;26:1991–1999. doi: 10.1523/JNEUROSCI.5055-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eugenín EA, et al. Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-γ and tumor necrosis factor-α. Proc. Natl Acad. Sci. 2001;98:4190–4195. doi: 10.1073/pnas.051634298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto T, Ochalski A, Hertzberg EL, Nagy JI. On the organization of astrocytic gap junctions in rat brain as suggested by LM and EM immunohistochemistry of connexin43 expression. J. Comp. Neurol. 1990;302:853–883. doi: 10.1002/cne.903020414. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto T, Vukelic J, Hertzberg EL, Nagy JI. Differential anatomical and cellular patterns of connexin43 expression during postnatal development of rat brain. Developmental Brain Res. 1992;66:165–180. doi: 10.1016/0165-3806(92)90077-A. [DOI] [PubMed] [Google Scholar]
- 67.Nagy JI, Patel D, Ochalski PA, Stelmack GL. Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience. 1999;88:447–468. doi: 10.1016/S0306-4522(98)00191-2. [DOI] [PubMed] [Google Scholar]
- 68.Elenes S, Rubart M, Moreno AP. Junctional communication between isolated pairs of canine atrial cells is mediated by homogeneous and heterogeneous gap junction channels. J. Cardiovasc Electrophysiol. 1999;10:990–1004. doi: 10.1111/j.1540-8167.1999.tb01270.x. [DOI] [PubMed] [Google Scholar]
- 69.Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J. Neurosci. 2001;21:1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pannasch U, Rouach N. Emerging role for astroglial networks in information processing: from synapse to behavior. Trends Neurosci. 2013;36:405–417. doi: 10.1016/j.tins.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 71.Díaz, E. F. et al. Connexin 43 hemichannels and pannexin‐1 channels contribute to the α‐synuclein‐induced dysfunction and death of astrocytes. Glia.67, 1598–1619 (2019). [DOI] [PubMed]
- 72.Konnova, E. A. & Swanberg, M. J. E. P. Animal models of Parkinson’s disease. Review. 83–106 (2018).
- 73.Rufer, M. et al. Regulation of connexin‐43, GFAP, and FGF‐2 is not accompanied by changes in astroglial coupling in MPTP‐lesioned, FGF‐2‐treated Parkisonian mice. J. Neurosci. Res. 46, 606–617 (1996). [DOI] [PubMed]
- 74.Zhang, S. et al. Reversal of rotenone-induced dysfunction of astrocytic connexin43 by opening mitochondrial ATP-sensitive potassium channels. Cell Mol. Neurobiol.31, 111–117 (2011). [DOI] [PMC free article] [PubMed]
- 75.Charron, G. et al. Astrocytosis in parkinsonism: considering tripartite striatal synapses in physiopathology? Front. Aging Neurosci. 6, 258 (2014). [DOI] [PMC free article] [PubMed]
- 76.Fujita, A. et al. Connexin 30 deficiency attenuates A2 astrocyte responses and induces severe neurodegeneration in a 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine hydrochloride Parkinson’s disease animal model. J. Neuroinflammation15, 1–20 (2018). [DOI] [PMC free article] [PubMed]
- 77.Rimkute, L. et al. Modulation of connexin-36 gap junction channels by intracellular pH and magnesium ions. Front. Physiol.9, 362 (2018). [DOI] [PMC free article] [PubMed]
- 78.Dragicevic E, Schiemann J, Liss B. Dopamine midbrain neurons in health and Parkinson’s disease: emerging roles of voltage-gated calcium channels and ATP-sensitive potassium channels. Neuroscience. 2015;284:798–814. doi: 10.1016/j.neuroscience.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 79.Surmeier DJ, Schumacker PT. Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease. J. Biol. Chem. 2013;288:10736–10741. doi: 10.1074/jbc.R112.410530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen X, et al. Potassium Channels: A Potential Therapeutic Target for Parkinson’s Disease. Neurosci. Bull. 2018;34:341–348. doi: 10.1007/s12264-017-0177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liss B, et al. Tuning pacemaker frequency of individual dopaminergic neurons by Kv4.3L and KChip3.1 transcription. EMBO J. 2001;20:5715–5724. doi: 10.1093/emboj/20.20.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vacher H, et al. Expanding the scorpion toxin α-KTX 15 family with AmmTX3 from Androctonus mauretanicus. Eur. J. Biochem. 2002;269:6037–6041. doi: 10.1046/j.1432-1033.2002.03294.x. [DOI] [PubMed] [Google Scholar]
- 83.Iacovitti L, et al. The hTH-GFP reporter rat model for the study of Parkinson’s disease. PLoS One. 2014;9:e113151. doi: 10.1371/journal.pone.0113151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chao H, et al. Lowered iPLA2γ activity causes increased mitochondrial lipid peroxidation and mitochondrial dysfunction in a rotenone-induced model of Parkinson’s disease. Exp. Neurol. 2018;300:74–86. doi: 10.1016/j.expneurol.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 85.Zhang, L., Zheng, Y., Xie, J. & Shi, L. Potassium channels and their emerging role in parkinson’s disease. Brain Res. Bull.160, 1–7, (2020). [DOI] [PubMed]
- 86.McCord MC, Aizenman E. Convergent Ca2+ and Zn2+ signaling regulates apoptotic Kv2. 1 K+ currents. Proc. Natl Acad. Sci. 2013;110:13988–13993. doi: 10.1073/pnas.1306238110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mitrofanis, J. Run in the Light: Exploring exercise and photobiomodulation in Parkinson’s disease. (Morgan & Claypool Publishers, 2019).
- 88.Shahmoradian SH, et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci. 2019;22:1099–1109. doi: 10.1038/s41593-019-0423-2. [DOI] [PubMed] [Google Scholar]
- 89.Tubert C, et al. Decrease of a Current Mediated by Kv1.3 Channels Causes Striatal Cholinergic Interneuron Hyperexcitability in Experimental Parkinsonism. Cell Rep. 2016;16:2749–2762. doi: 10.1016/j.celrep.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 90.Deffains M, Bergman H. Striatal cholinergic interneurons and cortico‐striatal synaptic plasticity in health and disease. Mov. Disord. 2015;30:1014–1025. doi: 10.1002/mds.26300. [DOI] [PubMed] [Google Scholar]
- 91.Rascol O, et al. Falls in ambulatory non-demented patients with Parkinson’s disease. J. Neural Transm. 2015;122:1447–1455. doi: 10.1007/s00702-015-1396-2. [DOI] [PubMed] [Google Scholar]
- 92.Tarpey PS, et al. Mutations in UPF3B, a member of the nonsense-mediated mRNA decay complex, cause syndromic and nonsyndromic mental retardation. Nat. Genet. 2007;39:1127–1133. doi: 10.1038/ng2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu P-L, Lee M, Huang T-T. Effectiveness of physical activity on patients with depression and Parkinson’s disease: A systematic review. PLoS One. 2017;12:e0181515. doi: 10.1371/journal.pone.0181515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zimprich A, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 95.Parain K, et al. Reduced expression of brain-derived neurotrophic factor protein in Parkinson’s disease substantia nigra. Neuroreport. 1999;10:557–561. doi: 10.1097/00001756-199902250-00021. [DOI] [PubMed] [Google Scholar]
- 96.Mayfield J, Blednov YA, Harris RA. Behavioral and genetic evidence for GIRK channels in the CNS: role in physiology, pathophysiology, and drug addiction. Int. Rev. Neurobiol. 2015;123:279–313. doi: 10.1016/bs.irn.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.da Silva TM, et al. Depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J. Affect. Disord. 2008;111:351–359. doi: 10.1016/j.jad.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 98.Conti B, et al. Uncoupling protein 2 protects dopaminergic neurons from acute 1,2,3,6-methyl-phenyl-tetrahydropyridine toxicity. J. neurochemistry. 2005;93:493–501. doi: 10.1111/j.1471-4159.2005.03052.x. [DOI] [PubMed] [Google Scholar]
- 99.Giladi N, et al. Risk factors for dementia, depression and psychosis in long-standing Parkinson’s disease. J. Neural Transm. 2000;107:59–71. doi: 10.1007/s007020050005. [DOI] [PubMed] [Google Scholar]
- 100.Ray NJ, et al. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson’s disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp. Neurol. 2008;213:108–113. doi: 10.1016/j.expneurol.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 101.Mela F, et al. Antagonism of metabotropic glutamate receptor type 5 attenuates l-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson’s disease. J. neurochemistry. 2007;101:483–497. doi: 10.1111/j.1471-4159.2007.04456.x. [DOI] [PubMed] [Google Scholar]
- 102.Zhang L, Zheng Y, Xie J, Shi L. Potassium channels and their emerging role in parkinson’s disease. Brain Res. Bull. 2020;160:1–7. doi: 10.1016/j.brainresbull.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 103.Zhang, X. et al. Reduced Circulating Levels of miR-433 and miR-133b Are Potential Biomarkers for Parkinson’s Disease. Front. Cell. Neurosci.11, 170 (2017). [DOI] [PMC free article] [PubMed]
- 104.Adelman JP, Maylie J, Sah P. Small-Conductance Ca2+-Activated K+ Channels: Form and Function. Annu. Rev. Physiol. 2012;74:245–269. doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- 105.Liu X-K, Wang G, Chen S-D. Modulation of the activity of dopaminergic neurons by SK channels: a potential target for the treatment of Parkinson’s disease? Neurosci. Bull. 2010;26:265–271. doi: 10.1007/s12264-010-1217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hurley MJ, Dexter DT. Voltage-gated calcium channels and Parkinson’s disease. Pharmacol. Therapeutics. 2012;133:324–333. doi: 10.1016/j.pharmthera.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 107.Yang F, et al. GDNF acutely modulates excitability and A-type K+ channels in midbrain dopaminergic neurons. Nat. Neurosci. 2001;4:1071–1078. doi: 10.1038/nn734. [DOI] [PubMed] [Google Scholar]
- 108.Cooper EC, Harrington E, Jan YN, Jan LY. M Channel KCNQ2 Subunits Are Localized to Key Sites for Control of Neuronal Network Oscillations and Synchronization in Mouse Brain. J. Neurosci. 2001;21:9529–9540. doi: 10.1523/JNEUROSCI.21-24-09529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martire M, et al. Involvement of KCNQ2 subunits in [3H]dopamine release triggered by depolarization and pre-synaptic muscarinic receptor activation from rat striatal synaptosomes. J. neurochemistry. 2007;102:179–193. doi: 10.1111/j.1471-4159.2007.04562.x. [DOI] [PubMed] [Google Scholar]
- 110.Shi L, et al. Peptide hormone ghrelin enhances neuronal excitability by inhibition of Kv7/KCNQ channels. Nat. Commun. 2013;4:1435. doi: 10.1038/ncomms2439. [DOI] [PubMed] [Google Scholar]
- 111.Peretz A, et al. Pre- and Postsynaptic Activation of M-Channels By a Novel Opener Dampens Neuronal Firing and Transmitter Release. J. Neurophysiol. 2007;97:283–295. doi: 10.1152/jn.00634.2006. [DOI] [PubMed] [Google Scholar]
- 112.Sander SE, Lemm C, Lange N, Hamann M, Richter A. Retigabine, a KV7 (KCNQ) potassium channel opener, attenuates l-DOPA-induced dyskinesias in 6-OHDA-lesioned rats. Neuropharmacology. 2012;62:1052–1061. doi: 10.1016/j.neuropharm.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 113.Lüscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat. Rev. Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mayfield, J., Blednov, Y. A. & Harris, R. A. In International Review of Neurobiology Vol. 123 (eds Paul A. Slesinger & Kevin Wickman) 279-313 (Academic Press, 2015).
- 115.Segura-Aguilar J, et al. Protective and toxic roles of dopamine in Parkinson’s disease. J. neurochemistry. 2014;129:898–915. doi: 10.1111/jnc.12686. [DOI] [PubMed] [Google Scholar]
- 116.Müller SK, et al. Lewy body pathology is associated with mitochondrial DNA damage in Parkinson’s disease. Neurobiol. Aging. 2013;34:2231–2233. doi: 10.1016/j.neurobiolaging.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 117.Chan CS, et al. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 118.Zhou Y, et al. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol. Neurodegeneration. 2016;11:28. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grassi D, et al. Identification of a highly neurotoxic α-synuclein species inducing mitochondrial damage and mitophagy in Parkinson’s disease. Proc. Natl Acad. Sci. 2018;115:E2634–E2643. doi: 10.1073/pnas.1713849115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zeng, X.-S., Geng, W.-S., Jia, J.-J., Chen, L. & Zhang, P.-P. Cellular and Molecular Basis of Neurodegeneration in Parkinson Disease. Front. Aging Neurosci.10, 109 (2018). [DOI] [PMC free article] [PubMed]
- 121.Birnbaum SG, et al. Structure and Function of Kv4-Family Transient Potassium Channels. Physiological Rev. 2004;84:803–833. doi: 10.1152/physrev.00039.2003. [DOI] [PubMed] [Google Scholar]
- 122.Serôdio P, Rudy B. Differential Expression of Kv4 K+ Channel Subunits Mediating Subthreshold Transient K+ (A-Type) Currents in Rat Brain. J. Neurophysiol. 1998;79:1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- 123.Haghdoost-Yazdi H, et al. Significant effects of 4-aminopyridine and tetraethylammonium in the treatment of 6-hydroxydopamine-induced Parkinson’s disease. Behavioural Brain Res. 2011;223:70–74. doi: 10.1016/j.bbr.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 124.Wolfart J, Neuhoff H, Franz O, Roeper J. Differential Expression of the Small-Conductance, Calcium-Activated Potassium Channel SK3 Is Critical for Pacemaker Control in Dopaminergic Midbrain Neurons. J. Neurosci. 2001;21:3443–3456. doi: 10.1523/JNEUROSCI.21-10-03443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hallworth NE, Wilson CJ, Bevan MD. Apamin-Sensitive Small Conductance Calcium-Activated Potassium Channels, through their Selective Coupling to Voltage-Gated Calcium Channels, Are Critical Determinants of the Precision, Pace, and Pattern of Action Potential Generation in Rat Subthalamic Nucleus Neurons In Vitro. J. Neurosci. 2003;23:7525–7542. doi: 10.1523/JNEUROSCI.23-20-07525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Deignan J, et al. SK2 and SK3 expression differentially affect firing frequency and precision in dopamine neurons. Neuroscience. 2012;217:67–76. doi: 10.1016/j.neuroscience.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim J-I, et al. Bee Venom Reduces Neuroinflammation in the MPTP-Induced Model of Parkinson’s Disease. Int. J. Neurosci. 2011;121:209–217. doi: 10.3109/00207454.2010.548613. [DOI] [PubMed] [Google Scholar]
- 128.Bosgraaf L, Van Haastert PJ. Roc, a Ras/GTPase domain in complex proteins. Biochimica et. biophysica acta. 2003;1643:5–10. doi: 10.1016/j.bbamcr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 129.Guaitoli, G. et al. Structural model of the dimeric Parkinson’s protein LRRK2 reveals a compact architecture involving distant interdomain contacts. Proc. Natl. Acad. Sci. USA113, E4357–E4366, (2016). [DOI] [PMC free article] [PubMed]
- 130.Paisán-Ruíz C, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 131.Chen L, Wu Z. Study on the Effects of EV Charging to Global Load Characteristics via Charging Aggregators. Energy Procedia. 2018;145:175–180. doi: 10.1016/j.egypro.2018.04.030. [DOI] [Google Scholar]
- 132.Greggio E. Role of LRRK2 kinase activity in the pathogenesis of Parkinson’s disease. Biochemical Soc. Trans. 2012;40:1058–1062. doi: 10.1042/BST20120054. [DOI] [PubMed] [Google Scholar]
- 133.Sheng, M., Sabatini, B. L. & Südhof, T. C. Synapses and Alzheimer’s disease. Cold Spring Harb. Perspect. Biol. 4, a005777 (2012). [DOI] [PMC free article] [PubMed]
- 134.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat. Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 135.Meade LB, Bearne LM, Sweeney LH, Alageel SH, Godfrey EL. Behaviour change techniques associated with adherence to prescribed exercise in patients with persistent musculoskeletal pain: Systematic review. Br. J. health Psychol. 2019;24:10–30. doi: 10.1111/bjhp.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fujiwara H, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat. cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 137.Brzezina, N., Kopainsky, B., Gerber, A. & Mathijs, E. System dynamics model-based policy evaluation tool: The case of organic farming policy in the EU. (2015).
- 138.Volpicelli-Daley LA, et al. G2019S-LRRK2 Expression Augments α-Synuclein Sequestration into Inclusions in Neurons. J. Neurosci. 2016;36:7415–7427. doi: 10.1523/JNEUROSCI.3642-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Longo Y, Coyne I, Joseph S. Development of the short version of the Scales of General Well-Being: The 14-item SGWB. Personal. Individ. Differences. 2018;124:31–34. doi: 10.1016/j.paid.2017.11.042. [DOI] [Google Scholar]
- 140.Qing J, et al. Antibody-based targeting of FGFR3 in bladder carcinoma and t(4;14)-positive multiple myeloma in mice. J. Clin. Investig. 2009;119:1216–1229. doi: 10.1172/JCI38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guerreiro R, et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Qing H, Wong W, McGeer EG, McGeer PL. Lrrk2 phosphorylates alpha synuclein at serine 129: Parkinson disease implications. Biochemical biophysical Res. Commun. 2009;387:149–152. doi: 10.1016/j.bbrc.2009.06.142. [DOI] [PubMed] [Google Scholar]
- 143.Lin, W., Zhang, M. & Wu, J. Simulation of low clouds from the CAM and the regional WRF with multiple nested resolutions. Geophys. Res. Lett.36, L08813 (2009).
- 144.Campoccia A, Dusonchet L, Telaretti E, Zizzo G. Comparative Analysis of Different Supporting Measures for the Production of Electrical Energy by Solar PV and Wind Systems: Four Representative European Cases. Sol. Energy. 2009;83:287–297. doi: 10.1016/j.solener.2008.08.001. [DOI] [Google Scholar]
- 145.Okochi M, et al. Constitutive phosphorylation of the Parkinson’s disease associated alpha-synuclein. J. Biol. Chem. 2000;275:390–397. doi: 10.1074/jbc.275.1.390. [DOI] [PubMed] [Google Scholar]
- 146.Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat. Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- 147.Waxman, E. A. & Giasson, B. I. Induction of Intracellular Tau Aggregation Is Promoted by α-Synuclein Seeds and Provides Novel Insights into the Hyperphosphorylation of Tau. J. Neurosci.31, 7604–7618 (2011). [DOI] [PMC free article] [PubMed]
- 148.Braithwaite J, et al. A four-year, systems-wide intervention promoting interprofessional collaboration. BMC Health Serv. Res. 2012;12:99. doi: 10.1186/1472-6963-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tenreiro S, Eckermann K, Outeiro TF. Protein phosphorylation in neurodegeneration: friend or foe? Front Mol. Neurosci. 2014;7:42–42. doi: 10.3389/fnmol.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Volpicelli-Daley LA, Kirik D, Stoyka LE, Standaert DG, Harms AS. How can rAAV-α-synuclein and the fibril α-synuclein models advance our understanding of Parkinson’s disease. J. neurochemistry. 2016;139:131–155. doi: 10.1111/jnc.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bieri G, et al. LRRK2 modifies α-syn pathology and spread in mouse models and human neurons. Acta Neuropathol. 2019;137:961–980. doi: 10.1007/s00401-019-01995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Marchant JS, Patel S. Two-pore channels at the intersection of endolysosomal membrane traffic. Biochemical Soc. Trans. 2015;43:434–441. doi: 10.1042/BST20140303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hockey LN, et al. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J. cell Sci. 2015;128:232–238. doi: 10.1242/jcs.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Eguchi, T. et al. LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis. Proc. Natl. Acad. Sci. USA.115, E9115–E9124, (2018). [DOI] [PMC free article] [PubMed]
- 155.Tong, Y. et al. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of α-synuclein, and apoptotic cell death in aged mice. Proc. Natl. Acad. Sci. USA107, 9879–9884, (2010). [DOI] [PMC free article] [PubMed]
- 156.Henry, C. S., Sheffield Morris, A. & Harrist, A. W. Family Resilience: Moving into the Third Wave. 64, 22–43, (2015).
- 157.Steger, M. et al. Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. Elife6, e31012 (2017). [DOI] [PMC free article] [PubMed]
- 158.Gitler, A. D. et al. The Parkinson’s disease protein α-synuclein disrupts cellular Rab homeostasis. 105, 145–150, (2008). [DOI] [PMC free article] [PubMed]
- 159.Gómez-Suaga P, et al. The VAPB-PTPIP51 endoplasmic reticulum-mitochondria tethering proteins are present in neuronal synapses and regulate synaptic activity. Acta Neuropathologica Commun. 2019;7:35. doi: 10.1186/s40478-019-0688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dinter, E. et al. Rab7 induces clearance of α-synuclein aggregates. J. Neurochem138, 758–774, (2016). [DOI] [PubMed]
- 161.Shih, Y.-L., Le, T. & Rothfield, L. Division site selection in Escherichia coli involves dynamic redistribution of Min proteins within coiled structures that extend between the two cell poles. Mol. Biol.100, 7865–7870, (2003). [DOI] [PMC free article] [PubMed]
- 162.Skibinski G, et al. Nrf2 mitigates LRRK2- and α-synuclein-induced neurodegeneration by modulating proteostasis. Proc. Natl Acad. Sci. USA. 2017;114:1165–1170. doi: 10.1073/pnas.1522872114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brady RO, Kanfer JN, Shapiro D. Metabolism of glucocerebrosides. Ii. Evidence of an enzymatic deficiency in gaucher’s disease. Biochemical biophysical Res. Commun. 1965;18:221–225. doi: 10.1016/0006-291X(65)90743-6. [DOI] [PubMed] [Google Scholar]
- 164.Stirnemann, J. et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci.18, 20441 (2017). [DOI] [PMC free article] [PubMed]
- 165.Oeda T, et al. Impact of glucocerebrosidase mutations on motor and nonmotor complications in Parkinson’s disease. Neurobiol. Aging. 2015;36:3306–3313. doi: 10.1016/j.neurobiolaging.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 166.Goker-Alpan O, et al. Parkinsonism among Gaucher disease carriers. J. Med. Genet. 2004;41:937–940. doi: 10.1136/jmg.2004.024455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sidransky E, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gan-Or Z, et al. Differential effects of severe vs mild GBA mutations on Parkinson disease. Neurology. 2015;84:880–887. doi: 10.1212/WNL.0000000000001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Taguchi YV, et al. Glucosylsphingosine Promotes α-Synuclein Pathology in Mutant GBA-Associated Parkinson’s Disease. J. Neurosci. 2017;37:9617–9631. doi: 10.1523/JNEUROSCI.1525-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mazzulli JR, et al. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Du Z-W, et al. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat. Commun. 2015;6:6626. doi: 10.1038/ncomms7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ysselstein D, et al. LRRK2 kinase activity regulates lysosomal glucocerebrosidase in neurons derived from Parkinson’s disease patients. Nat. Commun. 2019;10:5570. doi: 10.1038/s41467-019-13413-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Alcalay RN, et al. Glucocerebrosidase activity in Parkinson’s disease with and without GBA mutations. Brain. 2015;138:2648–2658. doi: 10.1093/brain/awv179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mazzulli, J. R., Zunke, F., Isacson, O., Studer, L. & Krainc, D. α-Synuclein–induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. 113, 1931–1936, 10.1073/pnas.1520335113 (2016). [DOI] [PMC free article] [PubMed]
- 175.Oaks AW, Frankfurt M, Finkelstein DI, Sidhu A. Age-dependent effects of A53T alpha-synuclein on behavior and dopaminergic function. PLoS One. 2013;8:e60378. doi: 10.1371/journal.pone.0060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Burbulla LF, et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Sci. (N. Y., N. Y.) 2017;357:1255–1261. doi: 10.1126/science.aam9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bedford C, Sears C, Perez-Carrion M, Piccoli G, Condliffe SB. LRRK2 Regulates Voltage-Gated Calcium Channel Function. Front Mol. Neurosci. 2016;9:35. doi: 10.3389/fnmol.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Antzoulatos E, Jakowec MW, Petzinger GM, Wood RI. Sex differences in motor behavior in the MPTP mouse model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2010;95:466–472. doi: 10.1016/j.pbb.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ni X, McGlinchey RP, Jiang J, Lee JC. Structural Insights into α-Synuclein Fibril Polymorphism: Effects of Parkinson’s Disease-Related C-Terminal Truncations. J. Mol. Biol. 2019;431:3913–3919. doi: 10.1016/j.jmb.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nam GE, et al. Metabolic syndrome and risk of Parkinson disease: A nationwide cohort study. PLOS Med. 2018;15:e1002640. doi: 10.1371/journal.pmed.1002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Athauda D, Foltynie T. Insulin resistance and Parkinson’s disease: A new target for disease modification? Prog. Neurobiol. 2016;145-146:98–120. doi: 10.1016/j.pneurobio.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 182.Piri H, et al. The Anti-Parkinsonism Effects of K(ATP) Channel Blockade in the 6-Hydroxydopamine-Induced Animal Model: The Role of Oxidative Stress. Basic Clin. Neurosci. 2017;8:183–192. doi: 10.18869/nirp.bcn.8.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Minaei A, Sarookhani MR, Haghdoost-Yazdi H, Rajaei F. Hydrogen sulfide attenuates induction and prevents progress of the 6-hydroxydopamine-induced Parkinsonism in rat through activation of ATP-sensitive potassium channels and suppression of ER stress. Toxicol. Appl. Pharmacol. 2021;423:115558. doi: 10.1016/j.taap.2021.115558. [DOI] [PubMed] [Google Scholar]
- 184.McFarthing K, et al. Parkinson’s disease drug therapies in the clinical trial pipeline: 2020. J. Parkinson’s Dis. 2020;10:757–774. doi: 10.3233/JPD-202128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Deb, R., Bhat, G., An, S., Shill, H. & Ogras, U. Y. Trends in Technology Usage for Parkinson’s Disease Assessment: A Systematic Review. 2021.2002.2001.21250939, 10.1101/2021.02.01.21250939 %J medRxiv (2021).
- 186.Thakur P, Nehru B. Long-term heat shock proteins (HSPs) induction by carbenoxolone improves hallmark features of Parkinson’s disease in a rotenone-based model. Neuropharmacology. 2014;79:190–200. doi: 10.1016/j.neuropharm.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 187.Thakur P, Nehru B. Inhibition of Neuroinflammation and Mitochondrial Dysfunctions by Carbenoxolone in the Rotenone Model of Parkinson’s Disease. Mol. Neurobiol. 2015;51:209–219. doi: 10.1007/s12035-014-8769-7. [DOI] [PubMed] [Google Scholar]
- 188.Liu, X. et al. General anesthetics have differential inhibitory effects on gap junction channels and hemichannels in astrocytes and neurons. Glia64, 524–536, (2016). [DOI] [PubMed]
- 189.Wang Y, Wu Z, Liu X, Fu Q. Gastrodin ameliorates Parkinson’s disease by downregulating connexin 43. Mol. Med Rep. 2013;8:585–590. doi: 10.3892/mmr.2013.1535. [DOI] [PubMed] [Google Scholar]
- 190.Freitas-Andrade M, Naus CC. Astrocytes in neuroprotection and neurodegeneration: The role of connexin43 and pannexin1. Neuroscience. 2016;323:207–221. doi: 10.1016/j.neuroscience.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 191.Blair HA, Dhillon S. Safinamide: A Review in Parkinson’s Disease. CNS drugs. 2017;31:169–176. doi: 10.1007/s40263-017-0408-1. [DOI] [PubMed] [Google Scholar]
- 192.Li, C. et al. Zonisamide for the Treatment of Parkinson Disease: A Current Update. Front. Neurosci.14, 574652 (2020). [DOI] [PMC free article] [PubMed]
- 193.Drouin-Ouellet, J. et al. Age-related pathological impairments in induced neurons derived from patients with idiopathic Parkinson’s disease. bioRxiv (2021). [DOI] [PMC free article] [PubMed]
- 194.Alexander A, et al. Recent expansions of novel strategies towards the drug targeting into the brain. Int. J. Nanomed. 2019;14:5895. doi: 10.2147/IJN.S210876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Huang R, et al. Neuroprotection in a 6‐hydroxydopamine‐lesioned Parkinson model using lactoferrin‐modified nanoparticles. J. gene Med.: A cross‐disciplinary J. Res. Sci. gene Transf. its Clin. Appl. 2009;11:754–763. doi: 10.1002/jgm.1361. [DOI] [PubMed] [Google Scholar]
- 196.Niu S, et al. Inhibition by multifunctional magnetic nanoparticles loaded with alpha-synuclein RNAi plasmid in a Parkinson’s disease model. Theranostics. 2017;7:344. doi: 10.7150/thno.16562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lang AE, Espay AJ. Disease modification in Parkinson’s disease: current approaches, challenges, and future considerations. Mov. Disord. 2018;33:660–677. doi: 10.1002/mds.27360. [DOI] [PubMed] [Google Scholar]
- 198.Bischoff SC, et al. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Coune, P. G., Schneider, B. L. & Aebischer, P. Parkinson’s Disease: Gene Therapies. Cold Spring Harbor Perspectives in Medicine2, a009431 (2012). [DOI] [PMC free article] [PubMed]
- 200.Rani K, et al. Neuronal exosomes in saliva of Parkinson’s disease patients: A pilot study. Parkinsonism Relat. Disord. 2019;67:21–23. doi: 10.1016/j.parkreldis.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 201.Rastogi, S. et al. The Evolving Landscape of Exosomes in Neurodegenerative Diseases: Exosomes Characteristics and a Promising Role in Early Diagnosis. Int. J. Mol. Sci.22, 440 (2021). [DOI] [PMC free article] [PubMed]
- 202.McCracken KW, Howell JC, Wells JM, Spence JR. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 2011;6:1920–1928. doi: 10.1038/nprot.2011.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Visanji NP, Brooks PL, Hazrati L-N, Lang AE. The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathologica Commun. 2013;1:2. doi: 10.1186/2051-5960-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annu. Rev. Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 205.Lim K-L, Tan JMM. Role of the ubiquitin proteasome system in Parkinson’s disease. BMC Biochem. 2007;8:S13–S13. doi: 10.1186/1471-2091-8-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hasegawa T, Sugeno N, Kikuchi A, Baba T, Aoki M. Membrane Trafficking Illuminates a Path to Parkinson’s Disease. Tohoku J. Exp. Med. 2017;242:63–76. doi: 10.1620/tjem.242.63. [DOI] [PubMed] [Google Scholar]
- 207.Pajares, M., et al. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications.Cells9, 1687 (2020). [DOI] [PMC free article] [PubMed]
- 208.Laird DW, Revel JP. Biochemical and immunochemical analysis of the arrangement of connexin43 in rat heart gap junction membranes. J. cell Sci. 1990;97:109–117. doi: 10.1242/jcs.97.1.109. [DOI] [PubMed] [Google Scholar]
- 209.Maeda S, et al. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 210.John SA, Revel JP. Connexon integrity is maintained by non-covalent bonds: intramolecular disulfide bonds link the extracellular domains in rat connexin-43. Biochemical biophysical Res. Commun. 1991;178:1312–1318. doi: 10.1016/0006-291X(91)91037-D. [DOI] [PubMed] [Google Scholar]
- 211.Laird DW, Lampe PD. Therapeutic strategies targeting connexins. Nat. Rev. Drug Discov. 2018;17:905–921. doi: 10.1038/nrd.2018.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sohl G, Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun. Adhes. 2003;10:173–180. doi: 10.1080/cac.10.4-6.173.180. [DOI] [PubMed] [Google Scholar]
- 213.Eiberger J, et al. Connexin genes in the mouse and human genome. Cell Commun. Adhes. 2001;8:163–165. doi: 10.3109/15419060109080717. [DOI] [PubMed] [Google Scholar]
- 214.Oertel WH. Recent advances in treating Parkinson’s disease. F1000Res. 2017;6:260–260. doi: 10.12688/f1000research.10100.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sweeney P, et al. Protein misfolding in neurodegenerative diseases: implications and strategies. Transl. Neurodegeneration. 2017;6:6. doi: 10.1186/s40035-017-0077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dey SK, Saini M, Prabhakar P, Kundu S. Dopamine β hydroxylase as a potential drug target to combat hypertension. Expert Opin. investigational drugs. 2020;29:1043–1057. doi: 10.1080/13543784.2020.1795830. [DOI] [PubMed] [Google Scholar]
- 217.Cereda E, et al. Efficacy of rasagiline and selegiline in Parkinson’s disease: a head-to-head 3-year retrospective case–control study. J. Neurol. 2017;264:1254–1263. doi: 10.1007/s00415-017-8523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mo J-J, et al. The effectiveness of creatine treatment for Parkinson’s disease: an updated meta-analysis of randomized controlled trials. BMC Neurol. 2017;17:105. doi: 10.1186/s12883-017-0885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang Y, et al. The efficacy and safety of pramipexole ER versus IR in Chinese patients with Parkinson’s disease: a randomized, double-blind, double-dummy, parallel-group study. Transl. Neurodegeneration. 2014;3:11. doi: 10.1186/2047-9158-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hobson DE, Pourcher E, Martin WR. Ropinirole andpramipexole, the new agonists. Can J Neurol Sci. 1999;26(Suppl 2):S27–33. doi: 10.1017/s0317167100000068. [DOI] [PubMed] [Google Scholar]
- 221.Park J, et al. Levodopa dose maintenance or reduction in patients with Parkinson's disease transitioning to levodopa/carbidopa/entacapone. Neurol. India. 2017;65:746–751. doi: 10.4103/neuroindia.NI_597_16. [DOI] [PubMed] [Google Scholar]
- 222.Pahwa R, et al. ADS-5102 (Amantadine) Extended-Release Capsules for Levodopa-Induced Dyskinesia in Parkinson Disease (EASE LID Study): A Randomized Clinical Trial. JAMA Neurol. 2017;74:941–949. doi: 10.1001/jamaneurol.2017.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Duits JH, Ongering MS, Martens HJM, Schulte PFJ. [Pimavanserin: a new treatment for the Parkinson’s disease psychosis] Tijdschr. Psychiatr. 2017;59:528–536. [PubMed] [Google Scholar]
- 224.Moore AH, et al. Non-Steroidal Anti-Inflammatory Drugs in Alzheimer’s Disease and Parkinson’s Disease: Reconsidering the Role of Neuroinflammation. Pharmaceuticals. 2010;3:1812–1841. doi: 10.3390/ph3061812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Farah A. Atypicality of atypical antipsychotics. Prim. Care Companion J. Clin. Psychiatry. 2005;7:268–274. doi: 10.4088/PCC.v07n0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhu Z-G, et al. The efficacy and safety of coenzyme Q10 in Parkinson’s disease: a meta-analysis of randomized controlled trials. Neurological Sci. 2017;38:215–224. doi: 10.1007/s10072-016-2757-9. [DOI] [PubMed] [Google Scholar]
- 227.Suárez-Rivero JM, et al. Mitochondrial Dynamics in Mitochondrial. Dis. Dis. 2017;5:1. doi: 10.3390/diseases5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Di Rocco A, Rogers JD, Brown R, Werner P, Bottiglieri T. S-adenosyl-methionine improves depression in patients with Parkinson’s disease in an open-label clinical trial. Mov. Disord. 2000;15:1225–1229. doi: 10.1002/1531-8257(200011)15:6<1225::AID-MDS1025>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 229.Nadanaciva S, et al. A high content screening assay for identifying lysosomotropic compounds. Toxicol. Vitr. 2011;25:715–723. doi: 10.1016/j.tiv.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 230.Davies GJ, Rhodes J, Calcraft BJ. Complications of carbenoxolone therapy. Br. Med. J. 1974;3:400–402. doi: 10.1136/bmj.3.5927.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Silberstein, S. et al. Tonabersat, a Gap-Junction Modulator: Efficacy and Safety in Two Randomized, Placebo-Controlled, Dose-Ranging Studies of Acute Migraineceph. Cephalalgi. 29, 17–27 (2009). [DOI] [PubMed]
- 232.Fang Y, Wang X. Ketamine for the treatment of refractory status epilepticus. Seizure. 2015;30:14–20. doi: 10.1016/j.seizure.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 233.Vandecasteele M, Glowinski J, Venance L. Connexin mRNA expression in single dopaminergic neurons of substantia nigra pars compacta. Neurosci. Res. 2006;56:419–426. doi: 10.1016/j.neures.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 234.Patel RS, Kamil S, Shah MR, Bhimanadham NN, Imran S. Pros and Cons of Marijuana in Treatment of Parkinson’s Disease. Cureus. 2019;11:e4813–e4813. doi: 10.7759/cureus.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Daniel NH, Aravind A, Thakur P. Are ion channels potential therapeutic targets for Parkinson’s disease? Neurotoxicology. 2021;87:243–257. doi: 10.1016/j.neuro.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 236.Venuto, C. S. et al. Isradipine plasma pharmacokinetics and exposure–response in early Parkinson’s disease. Ann. Clin. Transl. Neurol. 8, 603–612 (2021). [DOI] [PMC free article] [PubMed]
- 237.Deb, R., Bhat, G., An, S., Shill, H. & Ogras, U. Y. Trends in Technology Usage for Parkinson’s Disease Assessment: A Systematic Review. medRxiv, 2021.2002.2001.21250939, 10.1101/2021.02.01.21250939 (2021).
- 238.Pagano G, Niccolini F, Politis M. Imaging in Parkinson’s disease. Clin. Med (Lond.) 2016;16:371–375. doi: 10.7861/clinmedicine.16-4-371. [DOI] [PMC free article] [PubMed] [Google Scholar]