Abstract
Background
Psilocybin therapy has shown promise as a rapid-acting treatment for depression, anxiety, and demoralization in patients with serious medical illness (e.g., cancer) when paired with individual psychotherapy. This study assessed the safety and feasibility of psilocybin-assisted group therapy for demoralization in older long-term AIDS survivor (OLTAS) men, a population with a high degree of demoralization and traumatic loss.
Methods
Self-identified gay men OLTAS with moderate-to-severe demoralization (Demoralization Scale-II ≥8) were recruited from the community of a major US city for a single-site open-label study of psilocybin-assisted group therapy comprising 8–10 group therapy visits and one psilocybin administration visit (0·3–0·36 mg/kg po). Primary outcomes were rate and severity of adverse events, and participant recruitment and retention. The primary clinical outcome was change in mean demoralization from baseline to end-of-treatment and to 3-month follow-up assessed with a two-way repeated measures ANOVA. Trial registration: Clinicaltrials.gov (NCT02950467)
Findings
From 17 July 2017 to 16 January 2019, 18 participants (mean age 59·2 years (SD 4·4)) were enrolled, administered group therapy and psilocybin, and included in intent-to-treat analyses. We detected zero serious adverse reactions and two unexpected adverse reactions to psilocybin; seven participants experienced self-limited, severe expected adverse reactions. We detected a clinically meaningful change in demoralization from baseline to 3-month follow-up (mean difference -5·78 [SD 6·01], ηp2 = 0·47, 90% CI 0·21–0·60).
Interpretation
We demonstrated the feasibility, relative safety, and potential efficacy of psilocybin-assisted group therapy for demoralization in OLTAS. Groups may be an effective and efficient means of delivering psychotherapy pre- and post-psilocybin to patients with complex medical and psychiatric needs.
Funding
Carey Turnbull, Heffter Research Institute, NIMH R25 MH060482, NIH UL1 TR001872, River Styx Foundation, Saisei Foundation, Sarlo Foundation, Stupski Foundation, Usona Institute, US Department of Veterans Affairs (Advanced Neurosciences Fellowship and IK2CX001495).
Keywords: HIV/AIDS, Long-term survivors, Demoralization, Psilocybin, Trauma
Research in context.
Evidence before this study
For this protocol, we searched MEDLINE, Google Scholar, Erowid.org and the MAPS online bibliography up until November 2018 to review the literature on group therapy for people living with HIV (PLWH), existential psychotherapies for patients with serious medical illness, and psychedelic-assisted group therapy. This latter effort later expanded to a systematic review of psychedelic-assisted group therapy, which revealed promising, but non-systematic clinical evidence suggesting that psychedelic-assisted group therapy would be worth assessing in clinical trials as a treatment of alcohol use disorder and neuroses. Two recent systematic reviews found preliminary evidence of safety and efficacy of individual psychedelic-assisted psychotherapy for distress specifically in patients with serious medical illness. A recent meta-analysis (N = 117) of modern psilocybin therapy trials found promising signals of psilocybin therapy's efficacy in treating both depression and anxiety. Many early-phase studies exist of group psychotherapy for mood and trauma-related symptoms in PLWH, but a Cochrane review of psychosocial group interventions for adult PLWH found only a low certainty of evidence that these treatments improve depression, anxiety and coping in this population. Yet one of the largest of such studies (N = 361) by Heckman et al. (2013), which was not included in the Cochrane Review, found telephone-administered Supportive-Expressive Group Therapy for older PLWH to be significantly more effective at reducing depression symptoms than either an active comparator control or standard of care. No data are available specifically on psychotherapies for long-term AIDS survivors, nor on the use of psychedelics to treat distress in PLWH, however PLWH have previously been administered psilocybin in non-HIV-specific trials. Finally, meaning-centered group psychotherapy provides the best evidence for efficacy of a brief group psychotherapy for distress in patients with serious illness, with one trial (N = 253) demonstrating greater improvements in demoralization-related constructs (e.g., spiritual well-being, hopelessness and depression) compared to an active control condition.
Added value of this study
This pilot study is the first to demonstrate, following good clinical practices, the feasibility, relative safety, and potential efficacy of a psychedelic-assisted group therapy for distress in a population with serious medical illness. It is the first study, to our knowledge, specifically aimed at treating distress in long-term AIDS survivors. And it is the first study to attempt to treat demoralization with a medication-assisted psychotherapy.
Implications of all the available evidence
Psilocybin therapy merits further research as a brief, rapid-acting intervention for demoralized patients with serious medical illness, including PLWH, but the literature remains immature. Larger, randomized, controlled and well-blinded trials are needed. Future trials and clinical treatments involving psilocybin may consider using group psychotherapy, particularly for socially isolated and marginalized populations seeking help with mood- and trauma-related disorders.
Alt-text: Unlabelled box
1. Introduction
Demoralization is a form of existential suffering characterized by poor coping and a sense of helplessness, hopelessness, and a loss of meaning and purpose in life [1]. Demoralization is highly prevalent (13–53%) among patients with serious medical illness (e.g., advanced cancer and acquired immunodeficiency syndrome [AIDS], etc.) [2], [3], [4] and is associated with high physical symptom burden and poor quality of life [5,6]. Highly demoralized patients can screen negative for major depressive disorder (MDD); [3,4] and compared to MDD, demoralization is more strongly associated with suicidal ideation [6]. Demoralization-focused psychotherapies are available, but few have demonstrated efficacy when compared to an active control condition, and effect sizes have been modest [7–9].
While there are significant safety and ethical concerns with psychedelic medicines [10], recent trials of the serotonin 2A receptor (5HT2AR) agonist psilocybin, administered with adjunctive psychotherapy (henceforth “psilocybin therapy”), have found this intervention to be safe and to produce significant, enduring symptomatic improvement in a variety of psychiatric disorders, including demoralization and other forms of existential distress among patients with life-threatening cancer [11–14]. This may be a result of the increase in personal meaning attributed to one's lived experiences that has been demonstrated among both healthy volunteers [15] and patients [16,17] administered psilocybin. How psilocybin therapy may affect demoralization in other clinical populations remains unknown.
Early in the AIDS epidemic, demoralization was identified as a substantive clinical problem among the bereaved [18]; decades later, many older, long-term AIDS survivors (OLTAS) also suffer from demoralization. OLTAS are people diagnosed with human immunodeficiency virus (HIV) or AIDS prior to the clinical availability of protease inhibitors (∼1996), which were pivotal in transforming HIV from a terminal diagnosis to a chronic disease. Today over half of all people living with HIV (PLWH) in the United States of America are >50 years old [19]. And in San Francisco, California there are an estimated 2616 older long-term AIDS survivors (Personal communication, 2020: Ling Hsu, San Francisco Department of Public Health). The needs of OLTAS can be inferred from the data on samples of older PLWH, which indicate high rates of depression, anxiety, post-traumatic stress disorder (PTSD) and loneliness [[19], [20], [21]]. Key stakeholders in the OLTAS community have also identified renewed meaning in life and healthier coping with traumatic loss as two major mental health challenges (Personal communications, 2015: Jesus Guillen, Matt Sharp, and Vince Crisostomo). While meta-analysis has found a low certainty of evidence for group psychotherapies for PLWH [22], some data do support the efficacy of existential group psychotherapy for older PLWH [23], and yet no studies have examined how to treat distress specifically among OLTAS.
While psychedelic agents have long been used in traditional communal settings for rites of healing and prayer, and while psychedelics were administered with adjunctive group therapy to hundreds of participants prior to federal drug scheduling, [24] no modern clinical trials have investigated the safety or feasibility specifically of psilocybin-assisted group therapy. Yet mid-century [25] and modern [[26], [27], [28]] research on psychedelics emphasizes that an important therapeutic mechanism may be the enhanced capacity for interpersonal connection that patients can experience for days after drug administration. Indeed, the optimal method for delivering psilocybin therapy remains an open question.
We performed this pilot study to assess the safety, feasibility, and potential efficacy of psilocybin-assisted group therapy for demoralization in a population with serious, but not immediately life-threatening, medical illness—OLTAS men. Given the novel population, novel clinical approach, and the fact that the risks and benefits of psilocybin therapy remain poorly characterized, this study was designed to address clinical implementation issues, including participant recruitment, tolerability, and protocol adherence. Measures of clinical efficacy were exploratory.
2. Methods
2.1. Study design
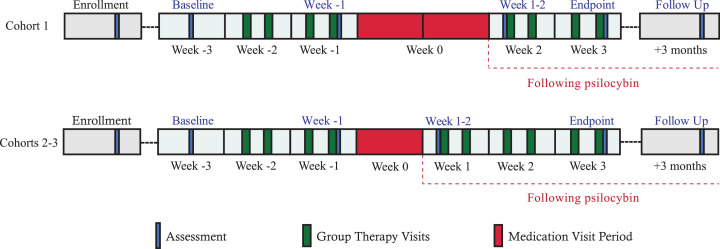
This was a single-arm, open-label, pilot study of psilocybin-assisted group therapy. Over seven weeks participants underwent three hours of individual psychotherapy, 12–15 h of group psychotherapy, and one eight-hour individual psilocybin administration session (Fig. 1). The study took place in an outpatient section of an urban academic medical center and was approved by the UCSF Institutional Review Board (CHR#15–17825), Food and Drug Administration, Research Advisory Panel of California, and Drug Enforcement Administration. Written informed consent was documented after the procedures had been fully explained, and the study was conducted according to Good Clinical Practices (NCT02950467).
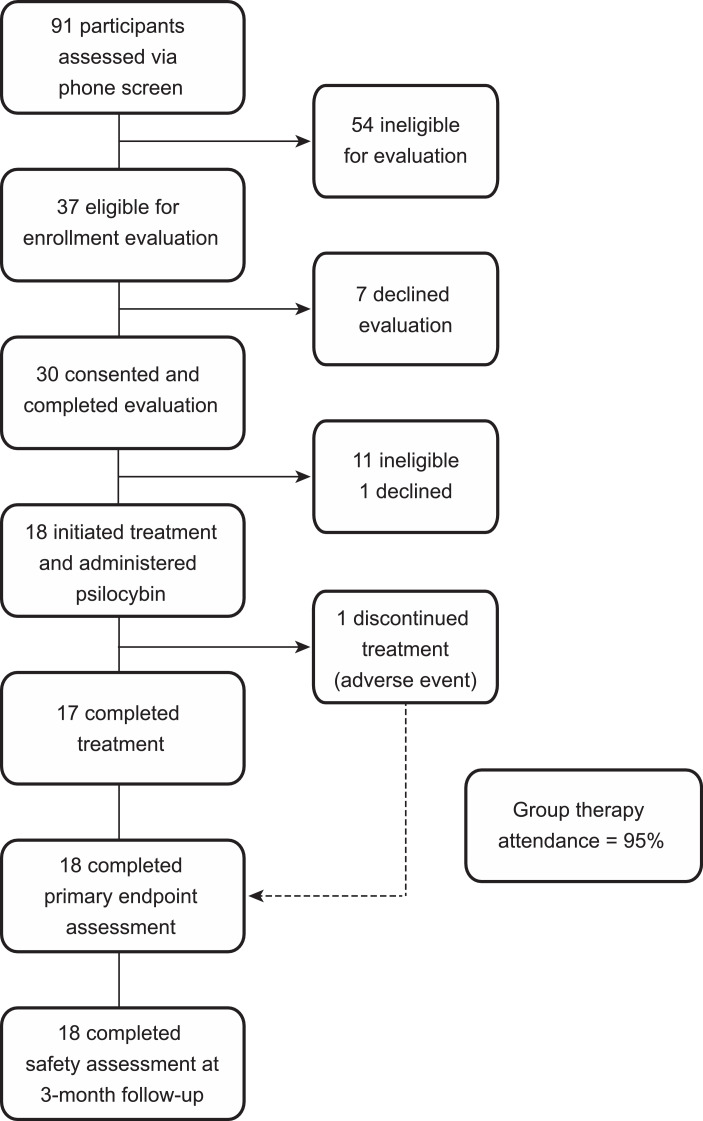
Fig. 1.
Trial profile.
The study comprised three sequential group therapy cohorts (each with n = 6 participants plus two therapists). The protocol was adapted in real-time in conjunction with feedback from the Data Safety Monitor and participant focus groups conducted after end-of-treatment. In response to feedback from Cohort 1 that more than four post-drug group therapy visits would be helpful, both subsequent cohorts had six post-drug group therapy visits. The study design (intervention characteristics and outcome measures), execution (clinician training and participant recruitment), analysis, and dissemination also benefitted greatly from consultation with OLTAS who were not participants enrolled in the study.
2.2. Participants
Participants were recruited by advertising in local HIV/AIDS clinics and service agencies. Inclusion criteria were: (1) Gay-identified, English-speaking cisgender men ≥50 years old and living with HIV; (2) Self-report of HIV diagnosis prior to the clinical availability of protease inhibitors (∼1996); and (3) Moderate-to-severe demoralization assessed by a Demoralization Scale-II (DS-II) [5] score of ≥8/32—a conservative cut-off based on published literature [3]. Participants found to be preliminarily eligible by phone screen were offered an in-person informed consent and enrollment assessment visit. Exclusion criteria were adapted from current standard criteria for psilocybin administration [29]. The study selected for gay-identified cis-gender men to create relatively homogenous group therapy cohorts to enhance a sense of trust and safety among participants in a brief period of time.
2.3. Procedures
2.3.1. Psychotherapy
The week prior to the first group session, participants met individually, for 90-minutes, with the two co-therapists assigned to their cohort to build trust and rapport, and to receive psychoeducation on group therapy and psilocybin. A second, two-hour individual psychotherapy visit occurred the day following the medication visit. During this session participants discussed their psilocybin experience with at least one of the clinicians who worked with them the day before, reviewing the content of the experience and attempting to generate meaning from the experience to apply to their day-to-day lives.
Ninety-minute group therapy sessions occurred twice per week. Group therapy was modeled on Brief Supportive Expressive Group Therapy (SEGT) [30] and its prior adaptations for PLWH [23,31]. SEGT is a palliative care-focused existential psychotherapy that prioritizes “here and now” processing, mutual support, “detoxifying death” and emotional expression. Our intervention required substantial modification to accommodate the psilocybin administration session; therefore, adherence to the SEGT model was not quantitatively assessed. For example, instead of the usual autohypnosis exercises used in traditional SEGT, our sessions began and ended with five minutes of breathing exercises and guided meditations focused on self-compassion and mindfulness, which served the dual purpose of emotional grounding for the group therapy sessions and training participants in techniques they could use to make the psilocybin sessions more tolerable.
2.3.2. Medication visits
Crystalline psilocybin was synthesized by Dr. David Nichols (Purdue University, Indiana). The UCSF Investigational Drug Service formulated the psilocybin in a gel cap to the nearest milligram at a dose of 0·3 mg/kg po for Cohort 1 and 0·36 mg/kg po for Cohorts 2 and 3. The pre-planned dose escalation was implemented after the 0·3 mg/kg dose was demonstrated to be well-tolerated by Cohort 1. A dose of 0·3 mg/kg had been shown to be safe in patients with cancer, [12,13] and a dose-response study in healthy volunteers suggested that optimum psilocybin dosing for eliciting a well-tolerated yet transcendent experience may be between 0·29 mg/kg and 0·43 mg/kg [32]. Each participant had at least one of their group co-therapists serve as one of the two guides for their medication visit.
For additional details regarding participants and procedures, see Supplemental Materials.
2.4. Outcomes
Feasibility was assessed by rates of recruitment and retention of enrolled participants. Qualitative feedback on the tolerability of study procedures was also collected through a focus group that occurred after end-of-treatment. No prespecified criteria were used to judge whether to proceed to a future definitive trial.
Safety was evaluated with multiple measures. Every study visit included an adverse events assessment interview and the Columbia Suicidality Severity Rating Scale (C-SSRS) interval interview. Adverse events assessments during the medication visit included spontaneous participant self-report, events observed by clinicians, and symptoms derived from the Challenging Experiences Questionnaire (ChEQ) [33] administered the next day. During psilocybin sessions, blood pressure and heart rate were assessed at regular intervals of 30 min for the first two hours, and then 60 min thereafter. At end-of-treatment, participant perceptions of benefits and harms from the intervention were assessed by a 7-point Likert scale (1= none at all; 7=extremely). The Schedule of Attitudes towards Hastened Death (SAHD) was administered at baseline, end-of-treatment and 3-month follow-up. The Montreal Cognitive Assessment (MoCA) was performed at enrollment and end-of-treatment. Because they were added after Cohort 1 completed the study, only Cohorts 2 and 3 completed the Alcohol Use Disorder Identification Test (AUDIT) and Drug Use Disorders Identification Test (DUDIT) at baseline and at the 3-month follow-up with a ‘last 3 month’ recall period.
The pre-specified primary clinical outcome measure was change in mean demoralization on the 16-item self-report DS-II with a ‘last week’ recall period. Data support using cut-off scores of 0–4 Low, 5–16 Medium and ≥17 High[3]; and an improvement of 2/32 points is considered clinically meaningful in palliative care patients [5]. Because the mental health needs of OLTAS are poorly characterized but likely multifaceted, [19–21] we employed several secondary clinical outcome measures (see Supplemental Materials). Notably, because our OLTAS consultants identified treatments for trauma and unresolved grief as a key need, we included both PTSD Checklist-5 (PCL-5) and Inventory of Complicated Grief-Revised (ICG-R). The DS-II and all secondary clinical outcome self-report measures were administered at least at baseline, end-of-treatment, and 3-month follow-up.
2.5. Data analysis
Based on the literature on group therapy for patients with serious medical illness, [34] we estimated that up to one third of participants might drop out of the trial. Thus, six participants were enrolled per group therapy cohort with the goal of having no fewer than four participants per cohort by end-of-treatment. Likewise, to ensure ≥12 participants would complete the intervention, 18 participants were enrolled.
Descriptive statistics assessed rates of recruitment and retention, baseline clinical characteristics, and frequency of adverse events. Given the small sample size and that few data were missing, we addressed missing data with single imputation (baseline observation carried forward) and then conducted intent-to-treat analyses. Change in clinical outcomes was assessed for most measures at least from baseline to end-of-treatment and to 3-month follow-up. Given the nested nature of group therapy outcomes data, [35] we performed these analyses with two-way repeated measures ANOVAs with participants nested within cohorts, with time as a random effect and cohort as a fixed effect. For individual model terms, we calculated and reported the standardized effect size partial eta squared (ηp2) with 90% confidence intervals [36]. To aid comparisons with trials that have reported standardized effect sizes in the Cohen's d family, we also report a bias-corrected standardized effect size for pre-post repeated measures (drm) with 95% confidence intervals calculated using ESCI [37] (however this effect size does not account for the nested nature of our data). All other analyses were performed in Microsoft Excel (v.2016) and Stata (v.16).
2.6. Role of the funding source
In this investigator-initiated study the funding sources had no role in study design, data collection, analysis or interpretation, or the writing of the report. The corresponding author had full access to all data and final responsibility for the decision to submit for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs or the United States Government.
3. Results
Eighteen eligible participants were enrolled from 17 July 2017 to 24 August 2018, and the last 3-month follow-up contact occurred in January 2019. (See Fig. 2) Participant demographics and baseline clinical characteristics are presented in Table 1. All 18 participants began the intervention, were administered study drug, completed the primary endpoint assessment and the 3-month follow-up safety evaluation, and were included in intent-to-treat analysis. Overall attendance of group therapy visits was 95%.
Table 2.
Adverse events.
| Serious Adverse Events (SAE) | ||||
|---|---|---|---|---|
| SAE Primary Term | # Participants (%), n = 18 | Highest severity observed | Expectedness | Relatedness to Psilocybin |
| DURING INTERVENTION | ||||
| Renal Cell Carcinoma | 1 (5·6%) | Severe | Unexpected | Unrelated |
| Recurrence, Metastatica | ||||
| Pneumothoraxa | 1 (5·6%) | Severe | Unexpected | Unrelated |
| DURING FOLLOW-UP | ||||
| Stimulant-induced psychosisb | 1 (5·6%) | Potentially life-threatening | Unexpected | Unrelated |
| Suicide attemptb | 1 (5·6%) | Potentially life-threatening | Unexpected | Unrelated |
| Cholecystitis | 1 (5·6%) | Severe | Unexpected | Unrelated |
| Medication Visit Adverse Events (AE) | ||||
| AE Primary Term | # Participants (%), n = 18 | Highest severity observed | Expectedness | Relatedness |
| Hypertension | Severe | Expected | Related | |
| Severe (SBP≥180 or DBP≥110 mmHg) | 4 (22·2%) | |||
| Moderate (160≤SBP<180 or 100≤DBP<109) | 8 (44·4%) | |||
| Anxiety/Anxiety Exacerbation (moderate-severe) | 8 (44·4%) | Severe | Expected | Related |
| Nausea | 6 (33·3%) | Severe | Expected | Related |
| Headache | 5 (27·8%) | Moderate | Expected | Related |
| Paranoia/Ideas of Reference | 4 (22·2%) | Mild | Expected | Related |
| Motor Agitation / Restlessness | 4 (22·2%) | Moderate | Expected | Related |
| Unsteady Gait / Ataxia | 4 (22·2%) | Moderate | Expected | Related |
| Tachycardia | 2 (11·1%) | Mild | Expected | Related |
| Thought Disorder | 1 (5·6%) | Moderate | Expected | Related |
| Urinary Incontinence | 1 (5·6%) | Moderate | Expected | Related |
| Visual Changes (complaint) | 1 (5·6%) | Mild | Expected | Related |
| Post-Medication Visit Psilocybin-Related Adverse Events | ||||
| AE Primary Term | # Participants | Highest severity | Expectedness | |
| Associated AEs | (%), n = 18 | observed | ||
| Headache | 8 (44·4%) | Mild | Expected | |
| Fatigue | 2 (11·1%) | Moderate | Expected | |
| Insomnia | 2 (11·1%) | Mild | Expected | |
| Anxiety Exacerbation | 1 (5·6%) | Severe | Expected | |
| Methamphetamine Relapse | Moderate | Unexpected | ||
| Post-traumatic Stress Flashback | 1 (5·6%) | Moderate | Unexpected | |
| Tinnitus, nausea, panic and insomnia | ||||
| Nausea | 1 (5·6%) | Mild | Expected | |
Adverse events were classified by the NIH DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events v2.0.
Same participant.
Same participant.
Fig. 2.
Timeline includes baseline assessment (“Week -3”), two weeks of group therapy with assessment after the fourth group therapy visit (“Week -1”), 1-2 weeks of individual psilocybin administration sessions (“medication visits”; “Week 0”), and then 2-3 weeks of group therapy. Medication visits for Cohort 1 occurred over a 2-week period; for Cohorts 2 and 3, all six medication visits occurred over a 1-week period, two per day in separate rooms. Post-drug participant-reported outcomes were collected on the same day for all participants in each cohort, hence there was a variable 3-day to 2-week period between drug administration and the next self-report assessment of symptoms, which occurred immediately after the fifth group therapy visit (“Week 1-2”). The primary endpoint was assessed immediately after the final group therapy visit (“end-of-treatment” a.k.a., “Week 3”). Final follow-up occurred 3 months after end-of-treatment.
Table 1.
Baseline characteristics of the intent-to-treat population.
| Characteristic | N = 18 | |
|---|---|---|
| Mean (SD) | Range | |
| Age (years) | 59·2 (4·4) | 50–66 |
| Year of HIV/AIDS/GRIDa Diagnosis | 1988 (4·8) | 1981–1996 |
| Palliative Performance Scale v2.0 | 92·8 (11·3) | 70–100 |
| Median (IQR) | Range | |
| Lifetime: People close to you who have died | 17·5 (7·25–30) | 2–100 |
| Lifetime: Use of a classic psychedelicb | 5 (3–23) | 0–200 |
| Years since last used a classic psychedelic | 20 (1–32) | 0·1–46 |
| N (%) | ||
| Race | ||
| Black or African American | 1 (5·6) | |
| Multiracial | 3 (16·7) | |
| White | 14 (77·7) | |
| Ethnicity | ||
| Hispanic/Latino | 1 (5·6) | |
| Civil status Single Married/Partnered Divorced/Separated |
8 (44·4) 8 (44·4) 2 (11·1) |
|
| Education College or more |
13 (72·2) |
|
| Annual income <$50,000/year |
10 (55·6) |
|
| Current SCID-5/SCID-5-PD diagnosis | ||
| Generalized anxiety disorder | 7 (38·9) | |
| Major depressive disorder | 5 (27·8) | |
| Panic disorder | 3 (16·7) | |
| Borderline personality disorder | 3 (16·7) | |
| No SCID-5/SCID-5-PD diagnosisc | 9 (50) | |
| Past week PCL-5 > 33 | 3 (16·7) | |
| Past medical historyd | ||
| Mood disorder | 11 (61·1) | |
| Anxiety disorder | 6 (33·3) | |
| Insomnia | 6 (33·3) | |
| Malignancy | 6 (33·3) | |
| Viral hepatitis | 6 (33·3) | |
| Chronic kidney disease | 5 (27·8) | |
| Chronic pain | 5 (27·8) | |
| Stimulant use disorder | 3 (16·7) |
GRID - Gay-related immune deficiency.
“Classic psychedelic” (e.g., LSD, mescaline, psilocybin, DMT) excludes entactogens (e.g., MDMA, MDA, etc.), cannabis products, and dissociative anesthetics (e.g., ketamine).
We assessed for the following SCID-5/SCID-5-PD diagnoses: Major depressive disorder, generalized anxiety disorder, panic disorder, obsessive-compulsive disorder, Cluster B personality disorders.
See Supplemental Materials for more details.
Absolute dosage of oral psilocybin ranged 22–32 mg with a mean of 27·1 mg (SD 3·1). No serious adverse events (SAEs) were attributed to psilocybin. During the medication visit, no participants required physical restraint or emergent medications for psychiatric or general medical concerns, and all psilocybin-related adverse events were self-limited and expected, although there were two unexpected post-medication visit psilocybin-reactions.
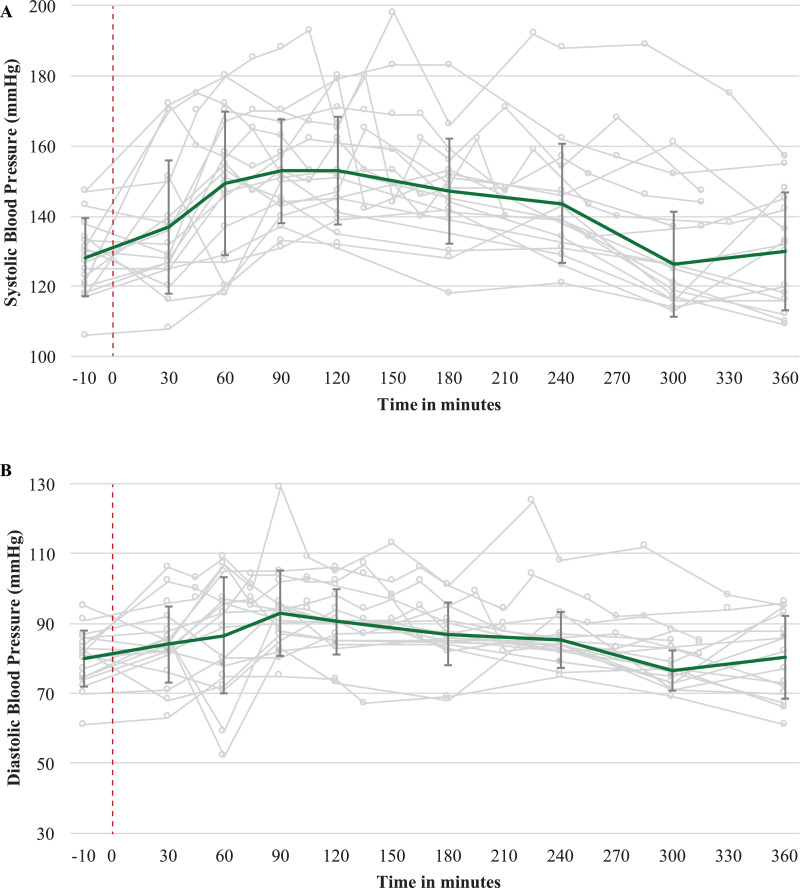
Adverse events occurring during the medication visit included both moderate and severe anxiety reactions (n = 8). Four participants experienced paranoia and one experienced transient thought disorder (i.e., dissociation with repeated self-talk). Four experienced self-limited, asymptomatic, severe hypertension that waxed and waned in conjunction with their anxiety symptoms; similarly, another eight participants experienced self-limited, moderate hypertension (See Fig. 3). The two participants with severe hypertension and severe anxiety also both met SCID-5-PD criteria for borderline personality disorder (BPD). Another participant, an HIV non-progressor who had never become ill from HIV, had severe nausea during the medication visit and experienced visual and tactile hallucinations of himself repeatedly vomiting and soiling his pants. He later reported that these hallucinations were related to his intention for the medication visit—to understand better the experiences of loved ones who had died during the AIDS epidemic.
Fig. 3.
Blood Pressure During Medication Visit. Error bars are 1 SD.
Fourteen participants experienced expected adverse reactions to psilocybin that were at least moderate in severity; seven participants experienced severe, but self-limited, expected adverse reactions. Two unexpected psilocybin reactions occurred: one participant, two days after his medication visit, experienced a moderate post-traumatic stress flashback during which he vividly re-experienced content that was not related to the psilocybin experience, but instead related to a sexual assault that happened years prior. The flashback was accompanied by nausea, tinnitus, panic and insomnia and left him unable to work for two days. Notably, he reported having a similar flashback years before when he started taking efavirenz, an antiretroviral and 5HT2AR agonist. Another participant with SCID-5 diagnosed generalized anxiety disorder, panic disorder, and BPD, in addition to decades of heavy polysubstance use, initially experienced significant anxiolysis after his medication visit. However, ten days later he reported severe anxiety, which he described as a deeply felt sense of being rejected by the other group members. He then had a lapse in his methamphetamine use (after having been sober from all substances for one month prior to the study) and withdrew from the study intervention but completed most subsequent assessments.
For details on safety measures, see Table 3. No change in suicidal ideation over time was detected with the C-SSRS, and no suicidal behavior was detected during the intervention. SAHD showed no meaningful change from baseline to 3-month follow-up. At end-of-treatment, participant-reported benefit and harm from the intervention were, on a 7-point Likert scale (1= none at all; 7=extremely), mean 6·61 (SD 0·6) and 1·17 (SD 0·38), respectively. Mean total ChEQ score was 37·11 (SD 26·0) or 28% of the maximum possible score. Mean MoCA showed no meaningful change from enrollment to end-of-treatment. Mean scores on AUDIT and DUDIT did not worsen from baseline to 3-month follow-up, suggesting that on average participation in the study did not lead participants to engage in more substance use.
Table 3.
Safety outcome measures.
|
ANOVA |
||||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Baseline Mean (SD) | Week −1 Mean (SD) | Day After Psilocybin Mean (SD) | Week 1–2 Mean (SD) | End-of-treatment Mean (SD) | 3-Month Mean (SD) | ηp2 (effect of time) | 90% CI (ηp2) |
| C-SSRS | 0·5 (0·99) | 0·11 (0·32) | 0 (0) | 0·11 (0·32) | 0·28 (0·75) | 0·12 | 0·0, 0·21 | |
| SAHD | 3·6 (3·3) | 1·7 (2·4) | 2·0 (2·2) | 2·7 (2·9) | 2·7 (2·4) | 0·25 | 0·07, 0·36 | |
| AUDIT | 6·0 (7·1) | 4·9 (6·5) | 0·40 | 0·03, 0·62 | ||||
| DUDIT | 6·4 (7·9) | 5·7 (5·8) | 0·05 | 0·0, 0·32 | ||||
| MoCA | 27·9 (1·4) | 28·3 (1·0) | 0·20 | 0·0, 0·44 | ||||
| Benefit | 6·6 (0·61) | |||||||
| Harm | 1·2 (0·38) | |||||||
| ChEQ | 37·1 (26·0) | |||||||
Baseline Observation Carried Forward was used to allow Intent-to-Treat Analysis for all enrolled participants. Legend: ηp2 = partial eta-squared; AUDIT = Alcohol Use Disorder Identification Test (last 3 months); Benefit = participant-reported harm from the intervention (1= none at all; 7=extremely); ChEQ = Challenging Experience Questionnaire; C-SSRS = Columbia Suicidality Severity Rating Scale; DUDIT = Drug Use Disorders Identification Test; Harm = participant-reported harm from the intervention (1= none at all; 7=extremely); MoCA = Montreal Cognitive Assessment; SAHD = Schedule of Attitudes Towards Hastened Death.
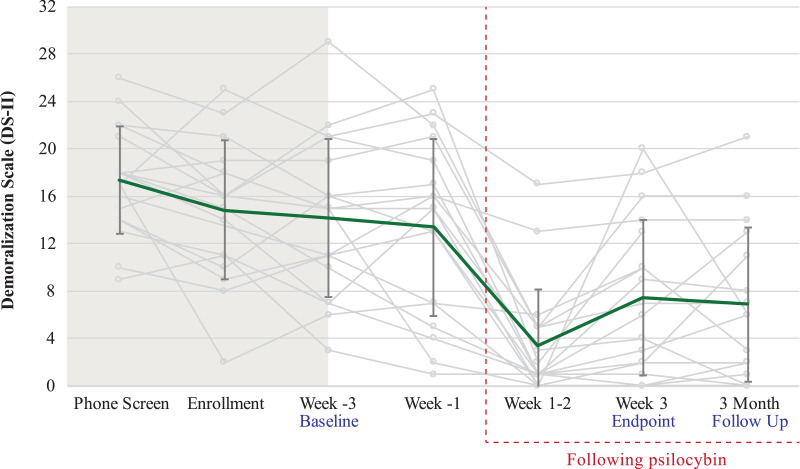
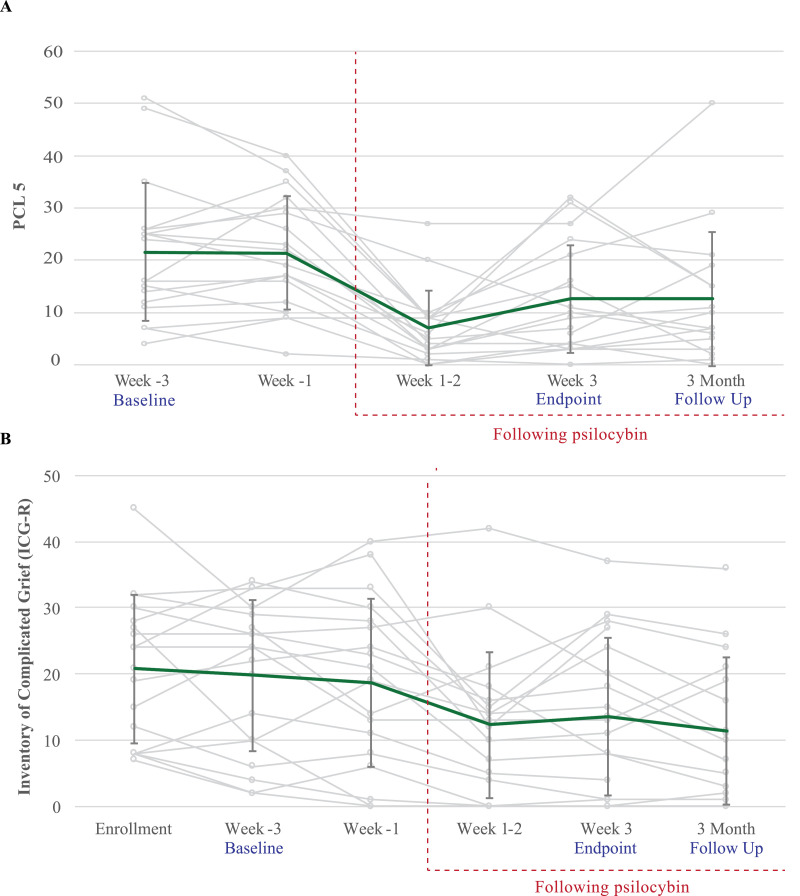
The pre-specified primary clinical outcome of change in demoralization showed a robust pre-post improvement from baseline to end-of-treatment (mean difference −6·67 [SD 6·51]) and from baseline to 3-month follow-up (mean difference −5·78 [SD 6·01]) with a standardized effect size of ηp2 = 0·47, 90% CI 0·21–0·60 for change over these three timepoints (Fig. 4). At end-of-treatment and 3-month follow-up, respectively, 88·9% and 66·7% of participants demonstrated at least a 2-point reduction in demoralization compared to baseline, and 50% and 33·3% demonstrated a > 50% reduction in demoralization compared to baseline. Change over time was also detected with all secondary clinical outcomes except for state anxiety (ηp2 = 0·16, 90% CI 0·0–0·32) and antiretroviral medication adherence (ηp2 = 0·08, 90% CI 0·0–0·22), the latter of which demonstrated a ceiling effect with 99% (SD 1·8) mean adherence at baseline. Notably, participants seemed to improve over time with respect to their symptoms of both PTSD (PCL-5, ηp2 = 0·27, 90% CI 0·05–0·43) and complicated grief (ICG-R, ηp2 = 0·45, 90% CI 0·19–0·58) (Fig. 5). See Supplemental Table S1 for secondary clinical outcomes data.
Fig. 4.
Primary clinical outcome (DS-II). Error bars are 1 SD. Mean change from Baseline (Week-3) to primary endpoint (Week 3) to 3-month follow-up has standardized effect size ηp2 = 0·47, 90% CI 0·21-0·60.
Fig. 5.
Selected secondary clinical outcomes. Error bars are 1 SD. A: PCL-5, B: ICG-R.
4. Discussion
This pilot study demonstrated the feasibility, relative safety, and potential efficacy of psilocybin-assisted group therapy in a sample of demoralized, gay-identified, OLTAS men—a population with heterogeneous and complex past medical and psychiatric histories. Our sample demonstrated greater psychiatric comorbidity than has so far been seen in the modern literature on psilocybin therapy. We detected zero psilocybin-related serious adverse events and two unexpected adverse reactions. There were moderate-to-severe, self-limited, expected adverse reactions in 14 of 18 participants—a relatively high rate of adverse events that we, in part, suspect is related to the clinical complexity of our study population. The primary clinical outcome, self-reported demoralization, demonstrated robust change from baseline to end-of-treatment and to 3-month follow-up. Our findings resemble those of recent psilocybin therapy trials that found the treatment to be well-tolerated in different psychiatric populations and to produce significant improvements in mood, anxiety, and existential distress lasting weeks-to-months after a single drug treatment [14]. The data are consistent with two systematic reviews [38,39] of individual psychedelic therapy for distress in patients with life-threatening illness, both of which found promising signals of safety and potential efficacy. Also, a recent meta-analysis of modern psilocybin therapy trials found that across four small studies (N = 69), pre-post measures of depression resulted in a pooled standardized effect size of Hedge's g = 1·38, 95% CI 0·78 to 1·99 [40]. For comparison, our pre-post standardized effect size estimate for demoralization was drm=0·97, 95% CI 0·43 to 1·57. Finally, our results compare favorably to those of a large (N = 253) trial of a brief (8-week), non-psychedelic, meaning-centered group psychotherapy for advanced cancer patients, which found a within-group Cohen's d = 0·54 for spiritual well-being, d = 0·53 for hopelessness, and d = 0·67 for depression [8].
Modern trials of psilocybin have only administered the drug to carefully-screened clinical populations with adjunctive psychotherapy, and always in an individual format. The logistics of providing group therapy for patients with serious illness can be taxing [34] and are only compounded by the addition of psychedelic drug administration. Nevertheless, our rates of recruitment and retention were high, demonstrating the feasibility of our intervention, with the caveat that our population was highly motivated (many self-referred to the study), and most were on disability or retired and had flexible schedules. Finally, our sample of 18 participants received a total of 472 face-to-face therapist-hours. Had we provided the same number of contact hours to all 18 participants with individual, instead of group psychotherapy, this would have equaled about 954 therapist-hours. Administering psilocybin with adjuvant group psychotherapy may yet prove to be an important means of delivering this treatment not only because of its potential for cost-savings but also because of group therapy's unique capacity to address social isolation, shame and stigma [24]. While it was evident to our clinical team that processing experiences of shame in the group setting was difficult for some participants, anecdotally, it also seemed helpful for some to receive validation from other group members that they were not alone with their struggles (e.g., being victims of sexual abuse). Furthermore, whereas some participants were slow to build rapport and trust with the study clinicians in the group setting, several participants reported that they were able to connect quickly with the other group members and that this peer support was helpful in tolerating and making use of the intervention. Nevertheless, two participants with BPD (see below) spontaneously reported persistent challenges with integrating their psilocybin experiences in the group setting.
In many countries, psilocybin is regulated as a Schedule I drug (or the equivalent). In the United States, a Schedule I drug has “high potential for abuse, no currently accepted medical use in treatment… and a lack of accepted safety for use under medical supervision [41].” The final clause of this definition, however, is at odds with our data and those in the published literature, [14] which suggest that psilocybin can be safely administered under appropriate medical supervision. Whereas we detected no serious adverse reactions to psilocybin, the number and severity of the non-serious adverse reactions observed in our sample do suggest that conducting psilocybin therapy with a population as complex as ours, and at this dose, should likely only be done with at least the level of medical screening, preparation and post-drug psychotherapy provided here.
Regarding cardiovascular risk, our participants displayed higher rates of moderate-to-severe hypertension during the psilocybin session than have been reported in other studies with clinical populations and a comparable dose [14]. While this is concerning given the high cardiovascular disease burden among OLTAS, [42] it is notable that severe hypertension in our trial seemed to co-occur with intense anxiety and self-resolved when the clinicians could calm the participant with verbal reassurance. However, one participant with a significant trauma history and who met criteria for BPD was intermittently severely hypertensive for four hours. Whereas he likely would have benefitted from a low dose benzodiazepine, during this period his paranoia precluded the clinicians from offering oral medications. His-blood pressure and anxiety eventually responded to our darkening the room and increasing the music volume. Although our two participants with significant trauma exposure and clinically active BPD symptoms reported very challenging medication visits, both also reported brief but notable clinical improvement from their baseline for 1–2 weeks post psilocybin (i.e., one no longer had regular anxiety-related vomiting before leaving his home, and one came out as gay to his long-time support group). Prior reports suggest that persons with borderline personality traits may have more difficulty tolerating psilocybin and other altered states of consciousness, [43,44] but also that challenging psilocybin experiences may nevertheless be beneficial [45]. Together, these data provide support for further investigations of psilocybin therapy not only for mood and anxiety disorders, but also, with the proper conditions in place, trauma-related disorders such as PTSD, BPD, and complicated grief.
Limitations inherent to open-label pilot studies, such as small sample size and absence of a control condition, were expected. The primary clinical outcome, demoralization, is little known beyond the fields of psycho-oncology and palliative care, however, severe demoralization does often co-occur with MDD [3]. A selection bias is notable from the fact that seven of our 18 participants had a lifetime history of classic psychedelic use ≥10 times, three even ≥100 times, and one had used entactogens (e.g., MDA) ‘hundreds’ of times; this could have biased our sample toward rating the experience as more tolerable or helpful than a psychedelic-naïve sample might. However, we did not find an association between higher lifetime psychedelic use and either total score on the Challenging Experience Questionnaire or the DS-II change scores (data not shown). Also, Cohort 1 had fewer group therapy visits and a lower drug dose than did Cohorts 2 and 3, but our sample is too small to meaningfully assess for a dose-response relationship of psychotherapy or drug to the clinical outcomes. Finally, stringent exclusion criteria are necessary to adequately blind participants to moderate-to-high dose psychedelics (e.g., excluding for any lifetime psychedelic use), [46] but we anticipated that such exclusion criteria would have rendered recruiting for a group therapy study with our population unfeasible. We therefore elected not to employ such stringent exclusion criteria, and hence we did not include a placebo-control arm given the high likelihood of functional unblinding.
The mental health needs and life experiences of OLTAS may be difficult to generalize to other populations given the degree of adverse life experiences many of these individuals have faced, including childhood abuse, severe stigma, multiple life-threatening medical conditions, and repeated exposure to traumatic loss and subsequent social isolation. The mean degree of demoralization experienced by our sample at baseline was moderate-to-severe and yet half of our participants did not meet SCID-5 criteria for any of the diagnoses assessed at enrollment. One third of our sample were survivors of life-threatening cancer, and indeed the psychological struggles of OLTAS can resemble those of cancer survivors [47]. To summarize, OLTAS may serve as a unique, enriched model of a distressed population that has confronted serious adversity, traumatic loss, and both acute and chronic life-limiting illness.
The open-label, uncontrolled, safety, feasibility and clinical outcomes measured here make a compelling case for controlled and well-blinded trials of psilocybin therapy (either with adjunctive group or individual psychotherapy) for demoralization, complicated grief, and other forms of distress experienced by patients with serious medical illness and traumatic stress. This study also highlights the need for further attention to the mental health needs of long-term AIDS survivors. As we advance towards the ‘end of AIDS,’ it is important to remember that for many the epidemic is far from over.
Contributors
BA and JW designed the study and BA coordinated the study, collected the data, and wrote the report. BA, JW, JM, MB and JD analyzed the data. AD was the lead study clinician and supervised with BA the psilocybin administration sessions. AD, RD and CS contributed to the creation of the group psychotherapy approach and served as group therapists. EE, GAL and AT contributed to data analysis and served as study clinicians. All authors critically revised the report and contributed intellectual content.
Declaration of interests
The authors declare no financial conflicts of interest related to for-profit entities. The following grants supported work on this trial: NIMH R25 MH060482, NIH UL1 TR001872, US Department of Veterans Affairs (Advanced Neurosciences Fellowship and IK2CX001495). BA and JW received philanthropic gifts from the following individuals and non-profit organizations to support this trial: Carey Turnbull, Heffter Research Institute, River Styx Foundation, Saisei Foundation, Sarlo Foundation, Stupski Foundation, Usona Institute. JW is conducting a trial sponsored by the non-profit Usona Institute. MB receives consulting fees from Beneath the Surface Foundation.
Acknowledgments
Acknowledgements
The following grants supported work on this trial: NIMH R25 MH060482, NIH UL1 TR001872, US Department of Veterans Affairs (Advanced Neurosciences Fellowship and IK2CX001495). BA and JW received philanthropic gifts from the following individuals and non-profit organizations to support this trial: Carey Turnbull, Heffter Research Institute, River Styx Foundation, Saisei Foundation, Sarlo Foundation, Stupski Foundation, Usona Institute. Drug was synthesized and supplied by Dr. David Nichols (Purdue University). We thank the study clinicians and study physicians who volunteered their time for this trial. In addition, thank you to Allison Coker for assistance with manuscript preparation, Bob Jesse for suggestions for the study concept and design, Erick Hung for serving as the Data Safety Monitor, and Hannah Tierney, Kelan Thomas, Phillip Perl, and Zach Matheson for serving as study staff and volunteers. We thank the OLTAS who participated in the study, and the participants who later reviewed and approved of this manuscript. Thank you to our OTLAS expert consultants Jesus Guillen, Matt Sharp and Vince Crisostomo.
Data sharing
Study protocol (with statistical analysis plan) and informed consent form are available online at clinicaltrials.gov. Individual de-identified participant data will be shared with other investigators on a case by case basis; please direct requests to the corresponding authors.
Funding
Carey Turnbull, Heffter Research Institute, NIMH R25 MH060482, NIH UL1 TR001872, River Styx Foundation, Saisei Foundation, Sarlo Foundation, Stupski Foundation, Usona Institute, US Department of Veterans Affairs (Advanced Neurosciences Fellowship and IK2CX001495).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100538.
Contributor Information
Brian T Anderson, Email: brian.anderson@ucsf.edu.
Joshua Woolley, Email: josh.woolley@ucsf.edu.
Appendix. Supplementary materials
References
- 1.Robinson S., Kissane D.W., Brooker J., Burney S. A systematic review of the demoralization syndrome in individuals with progressive disease and cancer: a decade of research. J Pain Symptom Manag. 2015;49:595–610. doi: 10.1016/j.jpainsymman.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Julião M., Nunes B., Barbosa A. Prevalence and factors associated with demoralization syndrome in patients with advanced disease: results from a cross-sectional Portuguese study. Palliat Support Care. 2016;14(5):468–473. doi: 10.1017/S1478951515001364. [DOI] [PubMed] [Google Scholar]
- 3.Ignatius J., De La Garza R., II Frequency of demoralization and depression in cancer patients. Gen Hosp Psychiatry. 2019;60(Sep –Oct):137–140. doi: 10.1016/j.genhosppsych.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Nanni M.G., Caruso R., Travado L., Ventura C., Palma A., Berardi A.M. Relationship of demoralization with anxiety, depression, and quality of life: a Southern European study of Italian and Portuguese cancer patients. Psycho‐Oncol. 2018;27(11):2616–2622. doi: 10.1002/pon.4824. [DOI] [PubMed] [Google Scholar]
- 5.Robinson S., Kissane D.W., Brooker J., Hempton C., Michael N., Fischer J. Refinement and revalidation of the demoralization scale: the DS‐II—External validity. Cancer. 2016;122(14):2260–2267. doi: 10.1002/cncr.30012. [DOI] [PubMed] [Google Scholar]
- 6.Vehling S., Kissane D.W., Lo C., Glaesmer H., Hartung T.J., Rodin G. The association of demoralization with mental disorders and suicidal ideation in patients with cancer. Cancer. 2017;123(17):3394–3401. doi: 10.1002/cncr.30749. [DOI] [PubMed] [Google Scholar]
- 7.Spiegel D. Existential psychotherapy for patients with advanced cancer: facing the future and the past. J Clin Oncol. 2015;33:2713–2714. doi: 10.1200/JCO.2015.62.2365. [DOI] [PubMed] [Google Scholar]
- 8.Breitbart W., Rosenfeld B., Pessin H., Applebaum A., Kulikowski J., Lichtenthal W.G. Meaning-centered group psychotherapy: an effective intervention for improving psychological well-being in patients with advanced cancer. J Clin Oncol. 2015;33:749–754. doi: 10.1200/JCO.2014.57.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kissane D.W., Lethborg C., Brooker J., Hempton C., Burney S., Michael N. Meaning and Purpose (MaP) therapy II: feasibility and acceptability from a pilot study in advanced cancer. Palliat Support Care. 2019;17(1):21–28. doi: 10.1017/S1478951518000883. [DOI] [PubMed] [Google Scholar]
- 10.Anderson B., Danforth A., Grob C. Psychedelic medicine: safety and ethical concerns. Lancet Psychiatry. 2020;70:829–830. doi: 10.1016/S2215-0366(20)30146-2. [DOI] [PubMed] [Google Scholar]
- 11.Grob C.S., Danforth A.L., Chopra G.S., Hagerty M., McKay C.R., Halberstadt A.L. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch. Gen. Psychiatry. 2011;68(1):71–78. doi: 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths R.R., Johnson M.W., Carducci M.A., Umbricht A., Richards W.A., Richards B.D. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181–1197. doi: 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross S., Bossis A., Guss J., Agin-Liebes G., Malone T., Cohen B. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30(12):1165–1180. doi: 10.1177/0269881116675512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas K., Malcolm B., Lastra D. Psilocybin-assisted therapy: a review of a novel treatment for psychiatric disorders. J Psychoactive Drugs. 2017;49(5):446–455. doi: 10.1080/02791072.2017.1320734. [DOI] [PubMed] [Google Scholar]
- 15.Preller K.H., Herdener M., Pokorny T., Planzer A., Kraehenmann R., Stämpfli P. The fabric of meaning and subjective effects in LSD-induced states depend on serotonin 2A receptor activation. Current Biol. 2017;27(3):451–457. doi: 10.1016/j.cub.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 16.Belser A.B., Agin-Liebes G., Swift T.C., Terrana S., Devenot N., Friedman H.L. Patient experiences of psilocybin-assisted psychotherapy: an interpretative phenomenological analysis. J Hum Psychol. 2017;57(4):354–388. [Google Scholar]
- 17.Swift T.C., Belser A.B., Agin-Liebes G., Devenot N., Terrana S., Friedman H.L. Cancer at the dinner table: experiences of psilocybin-assisted psychotherapy for the treatment of cancer-related distress. J Hum Psychol. 2017;57(5):488–519. [Google Scholar]
- 18.Martin J.L. Psychological consequences of AIDS-related bereavement among gay men. J Consult Clin Psychol. 1988;56(6):856–862. doi: 10.1037//0022-006x.56.6.856. [DOI] [PubMed] [Google Scholar]
- 19.Erenrich R., Seidel L., Brennan-Ing M., Karpiak S. HIV & aging in San Francisco: findings from the Research on Older Adults with HIV 2.0 San Francisco study. ACRIA. 2018 [Google Scholar]
- 20.John M., Greene M., Hessol N.A., Zepf R., Parrott A.H., Foreman C. Geriatric assessments and association with VACS index among HIV-infected older adults in San Francisco. J AIDS. 2016;72(5):534–541. doi: 10.1097/QAI.0000000000001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.High K.P., Brennan-Ing M., Clifford D.B., Cohen M.H., Currier J., Deeks S.G. HIV and aging: state of knowledge and areas of critical need for research. A report of the NIH Office of AIDS Research by the HIV and Aging Working Group. J. AIDS. 2012;60(Suppl 1):S1–S18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Heijden I., Abrahams N., Sinclair D. Psychosocial group interventions to improve psychological well‐being in adults living with HIV. Cochrane Database Syst Rev. 2017;(3) doi: 10.1002/14651858.CD010806.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heckman T.G. Supportive-Expressive and coping group teletherapies for HIV-infected older adults: a randomized clinical trial. AIDS Behav. 2013;19(9):3034–3044. doi: 10.1007/s10461-013-0441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trope A., Anderson B.T., Hooker A.R., Glick G., Stauffer C., Woolley J.D. Psychedelic-assisted group therapy: a systematic review. J Psychoactive Drugs. 2019;51(2):174–188. doi: 10.1080/02791072.2019.1593559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards W., Grof S., Goodman L., Kurland A. LSD-assisted psychotherapy and the human encounter with death. J Transpers Psychol. 1972;4:121–150. [Google Scholar]
- 26.Carhart-Harris R.L., Erritzoe D., Haijen E., Kaelen M., Watts R. Psychedelics and connectedness. Psychopharmacology (Berl.) 2018;235(2):547–550. doi: 10.1007/s00213-017-4701-y. [DOI] [PubMed] [Google Scholar]
- 27.Preller K.H., Vollenweider F.X. Modulation of social cognition via hallucinogens and “entactogens”. Front Psychiatry. 2019;10(881) doi: 10.3389/fpsyt.2019.00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forstmann M., Yudkin D.A., Prosser A.M.B., Heller S.M., Crockett M.J. Transformative experience and social connectedness mediate the mood-enhancing effects of psychedelic use in naturalistic settings. Proc Nat Acad Sci. 2020;117(5):2338–2346. doi: 10.1073/pnas.1918477117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson M.W., Richards W.A., Griffiths R.R. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22:603–620. doi: 10.1177/0269881108093587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Classen C., Diamond S., Soleman A., Fobair P., Spira J., Spiegel D. Stanford; 1993. Brief supportive-expressive group therapy for women with primary breast cancer. [Google Scholar]
- 31.Maldonado J., Gore-Felton C., Duran R., Diamond S., Koopman C., Spiegel D. Stanford; 1996. Supportive-expressive group therapy for people with HIV infection: a primer. [Google Scholar]
- 32.Griffiths R.R., Johnson M.W., Richards W.A., Richards B.D., McCann U., Jesse R. Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology (Berl.) 2011;218:649–665. doi: 10.1007/s00213-011-2358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrett F.S., Bradstreet M.P., Leoutsakos J.-.M.S., Johnson M.W., Griffiths R.R. The Challenging Experience Questionnaire: characterization of challenging experiences with psilocybin mushrooms. J Psychopharmacol. 2016;30(12):1279–1295. doi: 10.1177/0269881116678781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Applebaum A.J., Lichtenthal W.G., Pessin H.A., Radomski J.N., Simay Gökbayrak N., Katz A.M. Factors associated with attrition from a randomized controlled trial of meaning-centered group psychotherapy for patients with advanced cancer. Psychooncology. 2012;21:1195–1204. doi: 10.1002/pon.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldwin S.A., Murray D.M., Shadish W.R. Empirically supported treatments or type I errors? Problems with the analysis of data from group-administered treatments. J Consult Clin Psychol. 2005;73(5):924–935. doi: 10.1037/0022-006X.73.5.924. [DOI] [PubMed] [Google Scholar]
- 36.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4(863) doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cumming G. Routledge; 2016. Exploratory software for confidence intervals.http://tiny.cc/itnsroutledge [Google Scholar]
- 38.Ross S. Therapeutic use of classic psychedelics to treat cancer-related psychiatric distress. Int Rev Psychiatry. 2018;30(4):317–330. doi: 10.1080/09540261.2018.1482261. [DOI] [PubMed] [Google Scholar]
- 39.Reiche S., Hermle L., Gutwinski S., Jungaberle H., Gasser P., Majić T. Serotonergic hallucinogens in the treatment of anxiety and depression in patients suffering from a life-threatening disease: a systematic review. Progr Neuro-Psychopharmacol Biol Psychiatry. 2018;81:1–10. doi: 10.1016/j.pnpbp.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg S.B., Pace B.T., Nicholas C.R., Raison C.L., Hutson P.R. The experimental effects of psilocybin on symptoms of anxiety and depression: a meta-analysis. Psychiatry Res. 2020;284 doi: 10.1016/j.psychres.2020.112749. [DOI] [PubMed] [Google Scholar]
- 41.Johnson M.W., Griffiths R.R., Hendricks P.S., Henningfield J.E. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology. 2018;142:143–166. doi: 10.1016/j.neuropharm.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smit M., Brinkman K., Geerlings S., Smit C., Thyagarajan K., van Sighem A. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infectious Dis. 2015;15(7):810–818. doi: 10.1016/S1473-3099(15)00056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carhart-Harris R., Bolstridge M., Day C., Rucker J., Watts R., Erritzoe D. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl.) 2018;235(2):399–408. doi: 10.1007/s00213-017-4771-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Studerus E., Kometer M., Hasler F., Vollenweider F.X. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. Journal of Psychopharmacology. 2011;25:1434–1452. doi: 10.1177/0269881110382466. [DOI] [PubMed] [Google Scholar]
- 45.Carbonaro T.M., Bradstreet M.P., Barrett F.S., MacLean K.A., Jesse R., Johnson M.W. Survey study of challenging experiences after ingesting psilocybin mushrooms: acute and enduring positive and negative consequences. J Psychopharmacol. 2016;30(12):1268–1278. doi: 10.1177/0269881116662634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palhano-Fontes F., Barreto D., Onias H., Andrade K.C., Novaes M., Pessoa J. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655–663. doi: 10.1017/S0033291718001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shapiro C.L. Cancer survivorship. NEJM. 2018;379(25):2438–2450. doi: 10.1056/NEJMra1712502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.